Carboxylation
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide.[1] The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylation, especially when applied to the reaction of carbanionic reagents with CO2. More generally, carbonation usually describes the production of carbonates.[2]
Organic chemistry
[edit]Carboxylation is a standard conversion in organic chemistry.[3] Specifically carbonation (i.e. carboxylation) of Grignard reagents and organolithium compounds is a classic way to convert organic halides into carboxylic acids.[4]
Sodium salicylate, precursor to aspirin, is commercially prepared by treating sodium phenolate (the sodium salt of phenol) with carbon dioxide at high pressure (100 atm) and high temperature (390 K) – a method known as the Kolbe-Schmitt reaction. Acidification of the resulting salicylate salt gives salicylic acid.
Many detailed procedures are described in the journal Organic Syntheses.[5][6][7]
Carboxylation catalysts include N-Heterocyclic carbenes [8] and catalysts based on silver.[9]
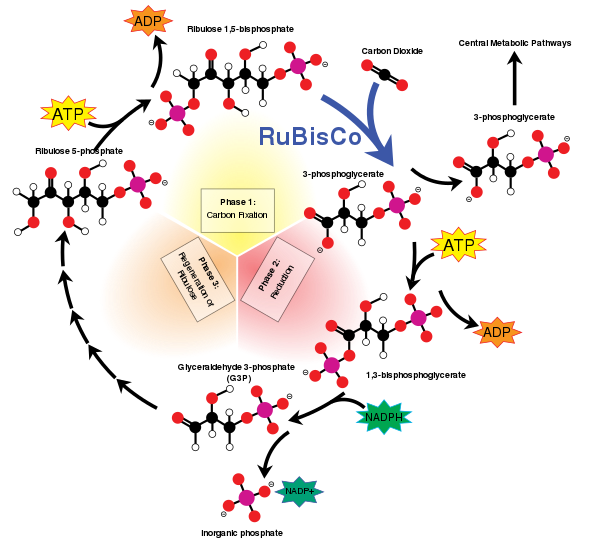
Carboxylation in biochemistry
[edit]Carbon-based life originates from carboxylation that couples atmospheric carbon dioxide to a sugar. The process is usually catalysed by the enzyme RuBisCO. Ribulose-1,5-bisphosphate carboxylase/oxygenase, the enzyme that catalyzes this carboxylation, is possibly the single most abundant protein on Earth.[10][11][12]

Many carboxylases, including Acetyl-CoA carboxylase, Methylcrotonyl-CoA carboxylase, Propionyl-CoA carboxylase, and Pyruvate carboxylase require biotin as a cofactor. These enzymes are involved in various biogenic pathways.[13] In the EC scheme, such carboxylases are classed under EC 6.3.4, "Other Carbon—Nitrogen Ligases".
Another example is the posttranslational modification of glutamate residues, to γ-carboxyglutamate, in proteins. It occurs primarily in proteins involved in the blood clotting cascade, specifically factors II, VII, IX, and X, protein C, and protein S, and also in some bone proteins. This modification is required for these proteins to function. Carboxylation occurs in the liver and is performed by γ-glutamyl carboxylase (GGCX).[14] GGCX requires vitamin K as a cofactor and performs the reaction in a processive manner.[15] γ-carboxyglutamate binds calcium, which is essential for its activity.[16] For example, in prothrombin, calcium binding allows the protein to associate with the plasma membrane in platelets, bringing it into close proximity with the proteins that cleave prothrombin to active thrombin after injury.[17]
See also
[edit]References
[edit]- ^ "Carboxylation: The introduction of a carboxyl group into a molecule or compound to form a carboxylic acid or a carboxylate; an instance of this."Oxford English Dictionary. Oxford University Press. 2018.
- ^ "Carbonation: Impregnation or treatment with carbon dioxide; conversion into a carbonate."Oxford English Dictionary. Oxford University Press. 2018.
- ^ Braunstein, Pierre; Matt, Dominique; Nobel, Dominique (August 1988). "Reactions of Carbon Dioxide with Carbon-Carbon Bond Formation Catalyzed by Transition-Metal Complexes". Chemical Reviews. 88 (5): 747–764. doi:10.1021/cr00087a003.
- ^ A. M. Appel; et al. (2013). "Frontiers, Opportunities, and Challenges in Biochemical and Chemical Catalysis of CO2 Fixation". Chem. Rev. 113 (8): 6621–6658. doi:10.1021/cr300463y. PMC 3895110. PMID 23767781.
- ^ Akira Yanagisawa; Katsutaka Yasue; Hisashi Yamamoto (1997). "Regio- and Stereoselective Carboxylation of Allylic Barium Reagents: (E)-4,8-Dimethyl-3,7-Nonadienoic Acid". Organic Syntheses. 74: 178. doi:10.15227/orgsyn.074.0178.
- ^ H. Koch; W. Haaf (1964). "1-Adamantanecarboxylic Acid". Organic Syntheses. 44: 1. doi:10.15227/orgsyn.044.0001.
- ^ W. Haaf (1966). "1-Methylcyclohexanecarboxylic Acid". Organic Syntheses. 46: 72. doi:10.15227/orgsyn.046.0072.
- ^ Zhang Liang (2013). "N-Heterocyclic carbene (NHC)–copper-catalysed transformations of carbon dioxide". Chemical Science. 4 (9): 3395. doi:10.1039/C3SC51070K.
- ^ K. Sekine; T. Yamada (2016). "Silver-catalyzed carboxylation". Chem. Soc. Rev. 45 (16): 4524–4532. doi:10.1039/C5CS00895F. PMID 26888406.
- ^ Dhingra A, Portis AR, Daniell H (April 2004). "Enhanced translation of a chloroplast-expressed RbcS gene restores small subunit levels and photosynthesis in nuclear RbcS antisense plants". Proc. Natl. Acad. Sci. U.S.A. 101 (16): 6315–20. Bibcode:2004PNAS..101.6315D. doi:10.1073/pnas.0400981101. PMC 395966. PMID 15067115.
(Rubisco) is the most prevalent enzyme on this planet, accounting for 30–50% of total soluble protein in the chloroplast;
- ^ Feller U, Anders I, Mae T (2008). "Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated" (PDF). J. Exp. Bot. 59 (7): 1615–24. doi:10.1093/jxb/erm242. PMID 17975207.
- ^ Raven, John A. (April 2013). "Rubisco: still the most abundant protein of Earth?". New Phytologist. 198 (1): 1–3. doi:10.1111/nph.12197. PMID 23432200.
- ^ "Biotin – Fact Sheet for Health Professionals". Office of Dietary Supplements, US National Institutes of Health. 8 December 2017. Retrieved 25 February 2018.
- ^ OMIM - gamma-glutamyl carboxylase, contributed by McKusick VA, last updated October 2004 [1]
- ^ Morris DP, Stevens RD, Wright DJ, Stafford DW (1995). "Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate". J. Biol. Chem. 270 (51): 30491–8. doi:10.1074/jbc.270.51.30491. PMID 8530480.
- ^ Hauschka PV, Lian JB, Gallop PM (1975). "Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue". Proc. Natl. Acad. Sci. U.S.A. 72 (10): 3925–9. Bibcode:1975PNAS...72.3925H. doi:10.1073/pnas.72.10.3925. PMC 433109. PMID 1060074.
- ^ Berg JM, Tymoczko JL, Stryer L. Biochemistry, 5th ed. New York: W. H. Freeman and Company, 2002.
