Antibody Applications
Overview
Antibodies are a key element of any immune system, but they also have a lot of uses for research applications. The following article describes the structure and function of antibodies, which is essential knowledge for researching biologics, antigen tests, and using them as a tool in Western blots, chromatin immunoprecipitation, and immunofluorescence.
Request a consultation
Working on antibody discovery or other high throughput applications you’d like to discuss? Your time is valuable and we’ll prioritize your inquiry. Click on “Request a consultation” to provide brief information about your project, and we’ll be in touch to discuss it ASAP.
Request a consultationThe uses for antibodies
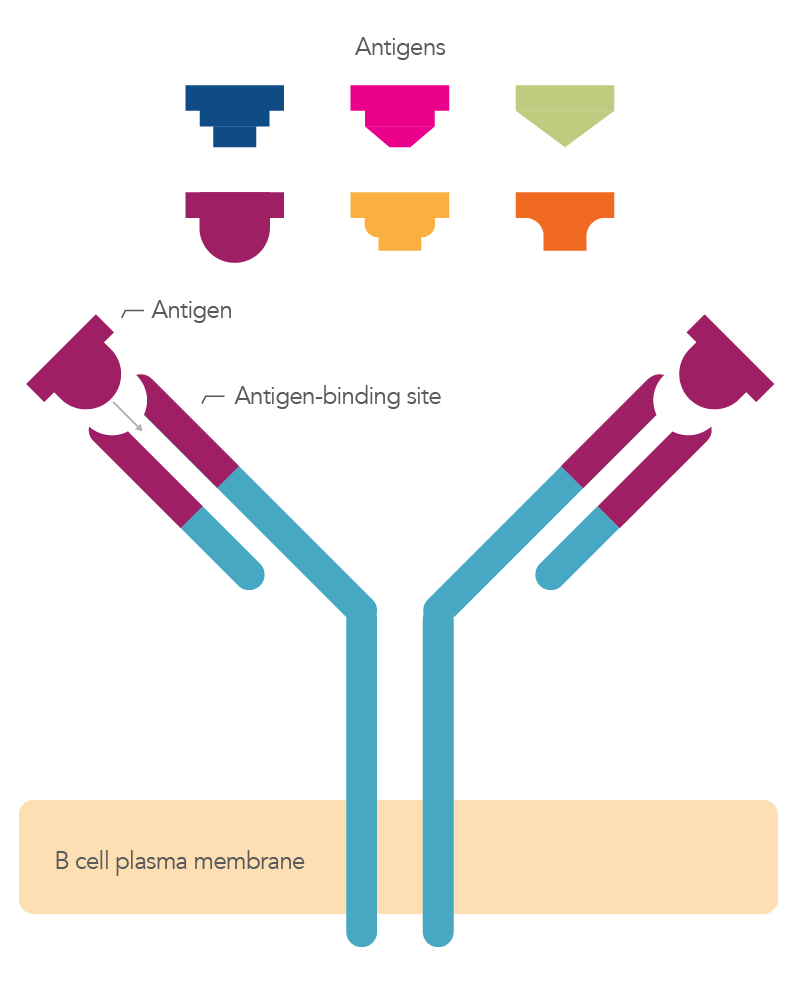
Antibodies are a specific type of protein. These specialized Y-shaped proteins bind to antigens, which can stimulate an immune response (Figure 1).
Today, antibodies are utilized in antigen testing kits and biologics. Antibodies are also an integral tool used in several research methods:
- Western blots
- Chromatin immunoprecipitation (ChIP)
- Enzyme-linked immunosorbent assay (ELISA)
Figure 1. Overview of an antibody
The function of antibodies
Antibodies are secreted into the blood and mucosa, which facilitates three biological functions:
- Neutralization occurs when the antibody binds to and inactivates the foreign substance, such as pathogens and toxins
- Activation of complement system facilitates the destruction of bacterial cells
- Opsonization of foreign substances facilitated by phagocytic cells
What is the anatomy of an antibody?
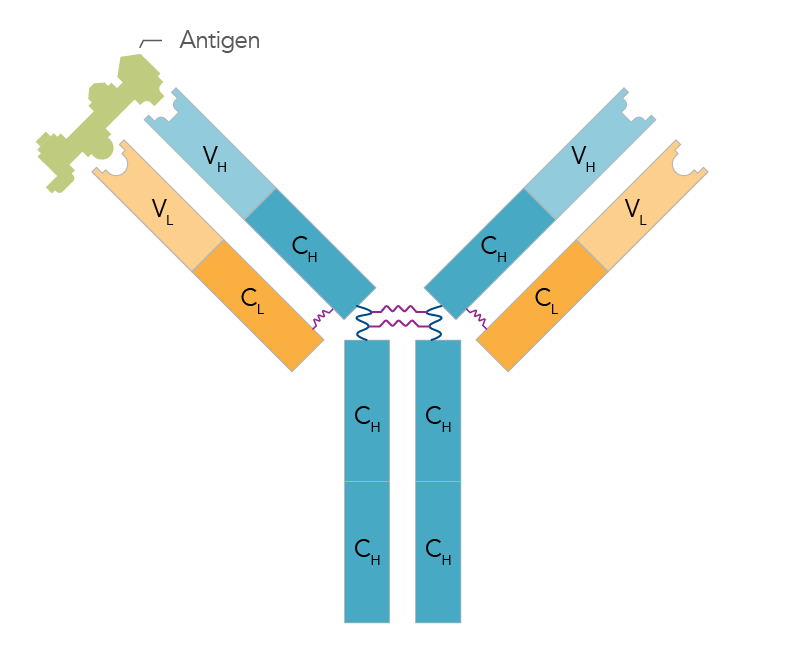
Antibodies are made up of two identical light chains (yellow) and two identical heavy chains (blue) (Figure 2).
Light chains: Have one variable domain (VL) and one constant domain (CL).
Heavy chains: Have one variable domain (VH) and three or four constant (CH) domains.
These variable domains are encoded by DNA. There are a countless number of combinations of these regions to create a diverse pool of antibodies with highly specific antigen-binding sites. Finding the most effective antigen-binding sequences can be used to research potential therapies and/or new diagnostic tests.
The difference between light and heavy chain is that the light chain is the small polypeptide subunit of an antibody while the heavy chain is the large polypeptide unit of an antibody.
The five types of antibodies
There are five types of heavy chain constant regions found in antibodies. Each antibody type is classified as IgG, IgM, IgA, IgD, or IgE. A summarized list of each antibody type and known function is described in Table 1.
Table 1. The five types of antibodies
| IgG |
| |
|---|---|---|
| IgM |
| |
| IgA |
| |
| IgD |
| |
| IgE |
|