Abstract
Electrochemical organic neuromorphic devices (ENODes) are rapidly developing as platforms for computing, automation, and biointerfacing. Resembling short- and long-term synaptic plasticity is a key characteristic in creating functional neuromorphic interfaces that showcase spiking activity and learning capabilities. This potentially enables ENODes to couple with biological systems, such as living neuronal cells and ultimately the brain. Before coupling ENODes with the brain, it is worth investigating the neuromorphic behavior of ENODes when they interface with electrolytes that have a consistency similar to brain tissue in mechanical properties, as this can affect the modulation of ion and neurotransmitter diffusion. Here, we present ENODEs based on different PEDOT:PSS formulations with various geometries interfacing with gel-electrolytes loaded with a neurotransmitter to mimic brain-chip interfacing. Short-term plasticity and neurotransmitter-mediated long-term plasticity have been characterized in contact with diverse gel electrolytes. We found that both the composition of the electrolyte and the PEDOT:PSS formulation used as gate and channel material play a crucial role in the diffusion and trapping of cations that ultimately modulate the conductance of the transistor channels. It was shown that paired pulse facilitation can be achieved in both devices, while long-term plasticity can be achieved with a tissue-like soft electrolyte, such as agarose gel electrolyte, on spin-coated ENODes. Our work on ENODe-gel coupling could pave the way for effective brain interfacing for computing and neuroelectronic applications.
Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Artificial synapses, utilizing electrochemical neuromorphic organic devices (ENODes), provide a potential avenue for addressing the Von Neumann bottleneck in hardware computing, owing to their ability to demonstrate non-volatile memory [1].
Moreover, envisioning also biointerfacing with the human brain for computing and restoring loss functionalities due to certain pathologies, organic bioelectronic and bioinspired devices hold promise as a more appropriate solution compared to inorganic counterparts, as they combine high biocompatibility limited immune response and operation in aqueous environment [1, 2].
Conductive polymers (CPs) feature ionic-electronic mixed conduction [3], low Young's modulus (∼kPa) [4], tunability through chemical functionalization [5], versatile processing [6, 7], and ease of integration into in vitro and in vivo platforms [2, 8, 9]. Therefore, CPs-based ENODes featuring electrochemical memory have been broadly studied to achieve artificial neurons and synapses through biosensing, computing and spiking circuits integration [1].
Among diverse ENODEs architectures, three terminal devices such as organic electrochemical transistors (OECTs) offer diverse advantages compared to two-terminal devices, like memristors, including their ion-to-electron transduction of signals [1, 10], operation in aqueous environment and modulation of conductance states based on ionic composition control (i.e. oxidation of neurotransmitters) [11, 12]. When ions interact with the bulk of CPs, they induce a modulation of the electrical current, through electrochemical doping or de-doping. Transconductance defines the efficiency of the ionic-to-electronic signal transduction (input signal) and is particularly high compared to the inorganic transistors [13]. Organic semiconductors are also preferable due to their biocompatibility and lower Young's Modulus [14]. Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) has been widely used to create tunable conductive states in ENODEs featuring both short- and long-term synaptic plasticity [15–17] and formation of neurohybrid connections with living neurons [18, 19], in a way that the biological cells can actively communicate with the artificial hardware. Advanced manufacturing techniques, such as printing, were employed to reduce the geometrical size of the OECTs, aiming to more refined device's figure of merit [20, 21], while keeping the process less time-consuming compared to conventional fabrication processes, i.e. photolithography. In several works, with aim of reducing problems due to leaking of aqueous electrolytes as well as increasing compactness of devices, gel electrolyte has been also integrated into OECTs. Moreover, to better integrate these electrolytes into transistor architectures [10, 22] and circuits [23] with high resolution and positioning and to exploit ionic migration and modulation, diverse gate electrodes materials and blends were investigated.
Furthermore, gel and solid electrolytes integration into OECTs has shown potential improvement in ionic conductivity of the electrolytes and the response speed of the devices when steady-state and transient response have been characterized [24, 25] while also allowing biological coupling [26].
To this end, it has been shown that biogel electrolytes consisting of an agarose-glycerol mixture, led to good electrical properties with high ON/OFF ratio and transconductance, as well as tunable mechanical properties [25]. Finally, a good control of the state‐retention property of biogel OECTs, demonstrated herein by employing them as artificial synapses with various synaptic functions [14], which would be relevant for interfacing ENODes directly with biological tissues and organs (i.e. skin, brain) to create functional hybrid communication [11]. To progress the development of this bidirectional neurohybrid interface, further studies of ENODes for various synaptic functions are still needed. In particular, the long-term plasticity is an important mechanism to achieve the bidirectional communication of artificial devices and neurons since complex computation tasks require memory retention. While short-term modulation reflects a temporary change in the doping level of the PEDOT:PSS, which can be restored within seconds after the removal of the gate stimulation [27], long-term modulation relies on different phenomena that change the doping level of the PEDOT:PSS in a way that cannot be reversed after the removal of the gate stimulation [15]. This leads to the memory capability of the device. In particular, this equilibrium can be disturbed by the production of more cations in solution due to redox reactions at the gate, such as the oxidation of neurotransmitters when a specific potential is applied to the gate terminal [18]. Neurotransmitter-based long term modulation was shown in biohybrid synapses based on PEDOT:PSS gate electrode with aqueous electrolyte [18] and more investigations with biogel electrolytes should be investigated.
In this work, we examined the behavior of biogel-based OECTs using PEDOT:PSS channel and gate as neuromorphic devices under various electrolyte viscosity and cations' diffusivity conditions.
Moreover, 0.6% agarose gel, commonly adopted as brain-phantoms to mimic mechanical properties of the neural system [28], were used as electrolyte to investigate the impact of the tissue-like viscosity on short term-plasticity. Finally, we characterized the impact on neurotransmitter-mediated long-term plasticity of the device, observing on the one hand a material-gate dependency on catecholamine neurotransmitters sensing and on the other hand a diffusivity-trapping of cations depending on the biogel electrolyte to tune the synaptic weight of the devices and to recapitulate mechanisms of long-term plasticity.
2. Materials and methods
2.1. ENODe fabrication with direct printing technique
Transistors were patterned using a printed circuit board (PCB) printer (Voltera NOVA). Glass substrates (Corning®) were sequentially washed with milliQ water, acetone and acetone, isopropyl alcohol (IPA) (Sigma-Aldrich, USA) and dried with a stream of nitrogen gas. Feedlines and contact pads were printed using silver ink (Ag Paste 520 EI, Chimet Spa, Italy), after homogenizing the ink in a planetary mixer (THINKY ARM-310) for 5 min and in the ultra-sound bath (CPX2800, Fisherbrand™) for 10 min. Thermal annealing was performed on hot plate at 130 °C for 20 min. PEDOT:PSS SV3 (Clevios SV3 STAB, Heraeus, Germany) was used for the gate and the channel of the transistors. According to the data supplied by the company, the SV3 ink contains propane-1,2-diol, 2-2'-oxydiethanol, and other additives at lower concentrations. The ink was homogenized in an ultra-sound bath for 10 min. After that, the channel of 2 × 5 mm and the gate of 5 × 10 mm were patterned with 1 mm between each other. The thickness of the polymeric layer is ∼1 µm [24]. Then, thermal annealing was performed on hot plate at 130 °C for 40 min. Polydimethylsiloxane (PDMS) (SYLGARD® 184, Dow Corning) was prepared by mixing silicone elastomer with the curing agent at a ratio of 10 : 1, for 3 min at 2000 rpm in a centrifugal mixer). It was printed over the Ag electrodes and then cured at 120 °C for 30 min on a hotplate. The same formulation of PDMS was poured and cured into PLA moulds for the preparation of walls used for the containment of electrolyte.
2.2. ENODe fabrication with spin coating technique
Transistors were fabricated on a 25 × 25 mm square glass substrate, with 10 × 10 mm indium tin oxide (ITO) square at each corner (Xinyan Technology Ltd). PEDOT:PSS (Clevios PH1000, Hereaus, Germany) aqueous solution was prepared by adding 5 vol.% ethylene glycol (Sigma-Aldrich, USA), 1 vol.% (3-glycidyloxypropyl)trimethoxysilane (Sigma-Aldrich, USA) and 0.02 vol.% dodecylbenzene sulfonic acid (Sigma-Aldrich, USA). A physical mask of Kapton tape has been placed on the glasses: two symmetrical stripes 7 × 17 mm (2 mm) apart were cut from the Kapton tape with the help of 3D-printed PLA aligners. The thickness of the polymeric layer is ∼100 nm [29]. After this step, glass substrates with Kapton were treated with oxygen plasma (Tecno-Service) for 10 min at 100 W. Subsequently the PEDOT:PSS solution was spin coated on the substrate at 2000 RPM for 2 min. Thermal annealing at 140 °C on hotplate was performed. PEDOT:PSS gate and channel patterning was completed with the peeling of the Kapton mask from the substate. Then the devices were immersed in milliQ water for 1 h to allow for the complete swelling of the PEDOT:PSS prior to further measurements.
2.3. Electrolyte solution preparation
Sodium Chloride NaCl (⩾99.5) (Sima-Aldrich, USA) was diluted in milli-Q water to prepare a stock of NaCl 1 M solution. Glycerol (Thermo Fisher Scientific GmbH) was mixed with milli-Q water and 1 M NaCl stock in different percentages. The electrolytes for the measurements were prepared in stocks of 10 ml or 5 ml: 0.1 M NaCl (1 ml of 1 M NaCl with 9 ml of milliQ water), glycerol 25 v/v% 0.1 M NaCl (1 ml of 1 M NaCl, 2,5 ml of glycerol, 6,5 ml of milliQ water), glycerol 50 v/v% 0.1 M NaCl (1 ml of 1 M NaCl, 5 ml of glycerol, 4 ml of milliQ water), glycerol 75 v/v% 0.1 M NaCl (1 ml of 1 M NaCl, 7,5 ml of glycerol, 1,5 ml of milliQ water). For the highest concentration of glycerol, 98%, a stock of 5 M NaCl was used (0.2 ml of 5 M NaCl and 9.8 ml of glycerol). PDMS was poured and cured into PLA molds for the preparation of wells with a surface of 4 mm × 15 mm used for the containment of the electrolyte, resulting in an active area of the OECTs channel of 4 mm × 7 mm (figure S6).
2.4. Agarose electrolyte
The phantom brain electrolyte was made by adding 0.06 g of agarose powder (CAS 9012–36-6) to 10 ml of 0.1 M NaCl no more than 3 h before to the tests. This mixture was microwaved on medium for 30 s after one to 2 min. It was then stirred, microwaved for an additional 10 s, then stirred once more. This process was continued until the mixture was uniform and the solution did not boil over. Subsequently, the mixture was poured in a 1 × 1 × 0.5 cm [3] Teflon mould and left to cool for 10 min to allow gelation. The Agarose gel was sliced, taken out of the mold, and applied to the device to take measurements.
2.5. Electrical measurements
The electrical measurements on printed ENODes were carried out with an Agilent B1500A source-measure unit under ambient conditions. The transfer curves were obtained at a constant drain voltage (Vds = −0.2 V). The gate voltage (Vgs) was swept from −0.2 V to 0.8 V (with 10 mV s−1 steps). Output curves were obtained by sweeping Vds from −0.6 V to 0.1 V. The transconductance was obtained as the first derivative of IDS with respect to Vgs. The transient response of transistors was obtained by keeping a fixed bias voltage Vds = −0.2 V, while applying square-wave pulses at the voltage gate. Each measurement consisted of six pulses with amplitude 0.3 V, pulse width 3 s and delay between pulses 9 s. For the paired-pulse facilitation (PPF) experiment the delay between 2 pulses were varying as multiple of the time constant of the device.
All electrical measurements on spin coated ENODes were carried out using a commercially available setup (Arkeo, Cicci Research, Italy) featuring two independent source measure units (SMUs) simultaneously. Pulsed operations were performed by keeping a fixed bias voltage Vds = −0.2 V, while applying square voltage pulses Vgs at the gate terminal. Each measurement consisted of six pulses with amplitude 0.3 V, pulse width 3 s and delay between pulses 9 s. The amplitude of the pulses was chosen considering the oxidation potential of the neurotransmitter. In particular, dopamine can be oxidized at 0.3–0.4 V. The timing was chosen considering the switching speed of the devices, which was quite slow. For the delay, a longer time was chosen to ensure that no overlapping of paired pulse facilitation phenomena (due to a small delay) and neurotransmitter-mediated long-term modulation would occur.
The electrochemical processes involved in doping/de-doping of the PEDOT:PSS channel can be described as the charging and discharging phases of this equivalent RC circuit, distinguished by a time constant (τ). This parameter has been calculated, by considering the time difference between the data point in which the pulsed voltage has been applied and the point in which the channel current of the OECTs has reached the 63.2% of the amplitude if the channel current at the last data point before the removal of the gate voltage.
2.6. Cyclic voltammetry
The electrochemical characterization of PEDOT:PSS films was performed by CV using Arkeo set-up (Cicci Research, Italy). The electrochemical potential values (from −0.2 V to 0.8 V) were measured with respect to a standard Ag/AgCl reference electrode, and a Pt wire was used as counter electrode. The working electrode was constituted by the PEDOT:PSS film and connected to the system though a spring probe, while the reference and counter electrodes were placed in the electrolyte well.
2.7. Viscosity measurements
The evaluation of the viscosity of the different electrolytes with different glycerol percentages was performed with a concentric cylinder configuration rheometer (MCR 501, Anton Paar). The measurements reported in the table S1 are referred to a shear rate equal to 1 s−1. The evaluation of the viscosity of the agarose gel was performed with a planar plates configuration rheometer (Kinexus, Malvern) and the elastic and the shear modulus was measured with different shear strain percentages from 10−2 % to 102% with an oscillation frequency of 1 Hz.
2.8. Neurotransmitter solution preparation and electrical measurements
Dopamine solution was obtained by dissolving dopamine hydrochloride in powder (Sigma-Aldrich, USA) in 0.1 M NaCl. Serotonin solution was obtained by dissolving serotonin hydrochloride (Sigma-Aldrich, USA) in 0.1 M NaCl. A stock of 10 mM of dopamine and serotonin was obtained and then dissolved to a final concentration of 30 µM in the specific electrolyte tested. 200 µl of neurotransmitter solution was inserted in the electrolyte placed on the transistor. For the measurements in agarose gel electrolyte, 1.6 µl of a 10 mM stock solution was injected in the agarose gel (volume of 200 µl). Each measurement consisted of 6 voltage pulses applied at the gate terminal. The channel conductance was calculated by dividing the channel current by Vds. Then, conductance variation (difference between values before and after the application of Vgs pulses) was represented as percentage.
At least N = 3 devices were measured.
2.9. Data analysis and statistics
All experimental data have been acquired and processed based on N = 3 independent experiments. Mean values and standard deviations of measurements was considered for the statistics. Data analysis was carried out through custom made Python scripts.
3. Results and discussion
First, two type of OECT devices were fabricated through spin coating and printing deposition techniques with different PEDOT:PSS blends as previously reported [24, 29]. Spin-coated ENODes were fabricated with gate and channel both with a dimension of 7 × 17 (width × length) mm and a thickness of ∼100 nm [29] whereas printed devices had a gate of 5 × 10 mm, a channel of 2 × 5 (width × length) mm, and thickness of ∼1 µm [24].
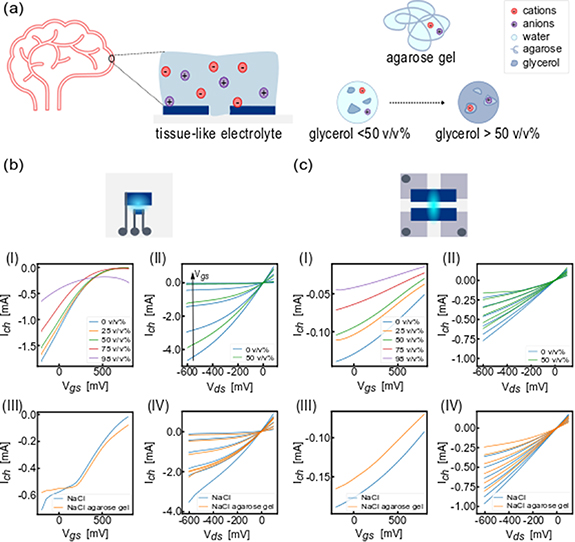
The steady-state behavior of these devices has been characterized with different electrolyte compositions including liquid and gel electrolytes (figure 1, gate voltages Vgs = −0.2 V–0.8 V and drain voltage Vds = −0.6 V–0.1 V). 0.1 M NaCl in glycerol-water mixtures with increasing concentrations ([0,25,50,98] v/v%) were used. The hydrogen bonding between glycerol and water molecules contributing to the electrolyte's viscosity and the diffusivity of cations strongly depend on the concentration of glycerol in the mixture [30] (figure 1(a)). The resulting electrolyte viscosities are reported in table s1.
Figure 1. ENODes steady-state characterization with different electrolytes. (a), Schematics of structure of electrolytes used with the addition of 0.1 M NaCl: water-glycerol mixture with different concentrations and 0.6% agarose hydrogel 'phantom brain'. (b), Electrical characterization of printed ENODes with PEDOT:PSS SV3: transfer (b-I) and output curves (b-II) when different concentrations of glycerol are added in the electrolyte; transfer (b-III) and output curves (b-IV) when 0.6% agarose hydrogel/0.1 M NaCl was used as electrolyte. (c), Electrical characterization of spin coated ENODes based on spin coated PEDOT:PSS: transfer (c-I) and output curves (c-II) when different concentrations of glycerol were added in the electrolyte; transfer (c-III) and output curves (c-IV) when 0.6% agarose hydrogel 0.1 M NaCl was used as electrolyte. All the measurements were repeated three times, the curves reported are examples.
Download figure:
Standard image High-resolution imageTransfer curves for different electrolytes were recorded at Vds = −0.2 V, output characteristics were measured within the range mentioned above. In figure 1 output curves in all electrolytes are reported for all considered voltage. Transfer (figures 1(bI)–(cI)) and output (figures 1(bII)–(cII)) curves show that PEDOT:PSS SV3 printed ENODes have higher channel currents and transconductances values compared to spin-coated PEDOT:PSS devices for both type of electrolytes, likely due to the higher thickness of transistor channel [31] or the difference in the blends of PEDOT:PSS. However, both devices featured a current decrease for increasing glycerol concentration, which can be explained by hindering of ion migration toward the transistor channel in electrolyte with increased viscosity [32]. Moreover, the variation of maximum transconductance value also depended on the electrolyte composition: devices featured a transconductance decrease from ∼80 µS to ∼25 µS (∼70% loss) in spin-coated devices and from 1.75 mS to 0.45 mS (∼75% loss) in printed devices with water- based 0.1 M NaCl and 0.1 M NaCl in 98% glycerol electrolytes. Moreover, when 98 v/v% glycerol is present in the electrolyte, the increase of the voltage gate does not reflect a continuous decrease of the channel current, and this could be related to a very low diffusivity of ions inside the channel.
The steady-state measurements were then performed with a biogel electrolyte composed of 0.6% agarose gel including 0.1 M NaCl featuring a viscosity of 3 kPa (figure S2) and allowing for a higher diffusivity of molecules compared to glycerol, thanks to the presence of pores among the polymeric chains [33, 34]. Both ENODes types showed comparable transconductance values while operating with water-based and agarose electrolytes (figures 1 (bIII)–(cIII)), suggesting that the modulation of the transistor channel current was governed by diffusive processes in the electrolyte with a similar capacitive in the electrolyte, reflecting a similar ion-electronic coupling at the interface with organic polymer.
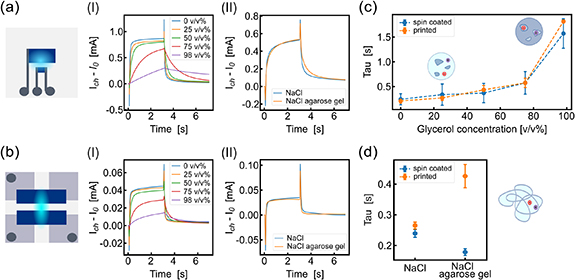
Then, short term plasticity of ENODes was characterized by considering the transient response to an individual voltage pulse (Vgs) applied at the gate electrode. The channel current of the devices was monitored while applying Vgs = 0.3 V for 3 s and keeping Vds = −0.2 V. While the potential was turned ON and cations were injected from the electrolyte, the polymeric channel was de-doped and the device turned OFF. The variation of channel current during de-doping decreased for increasing glycerol concentrations, suggesting a stronger capacitive effect in the electrolyte of both devices compared to the aqueous electrolyte (figures 2(aI)–(bI)). In contrast, this phenomenon was not noticeable in the agarose electrolyte, where the transient behavior was similar to the liquid electrolyte case (figures 2(aII)–(bII)). This could be ascribed to the high diffusivity of cations in the 0.1 M NaCl solution inside the 0.6% agarose gel [35], when compared to high concentrations of glycerol that strongly impacts the ions migration in aqueous mixtures, even if its viscosity is lower.
Figure 2. Transient behavior and short-term plasticity of ENODes with different electrolytes. (a), Channel current (with respect to the initial value I0) of ENODe based on PEDOT:PSS SV3 when a voltage pulse of 0.3 V was applied to the gate with 0.1 M NaCl and different glycerol concentrations ([0,25,50,75,98] v/v%) (a-I) and with 0.1 M NaCl agarose gel (a-II). (b), Channel current (with respect to the initial value I0) of spin coated ENODe when a voltage pulse of 0.3 V was applied to the gate with 0.1 M NaCl and different glycerol concentrations ([0,25,50,75,98] v/v%) (b-I) and with 0.1 M NaCl/agarose gel (b-II). (c), Time constant (tau, τ) of the ionic circuit between the gate and the channel of the ENODes when a voltage pulse of 0.3 V was applied to the gate with 0.1 M NaCl and different glycerol concentrations ([0,25,50,75,98] v/v%). (N = 3) (d), Time constant (tau, τ) of the ionic circuit between the gate and the channel of the ENODEs when a voltage pulse of 0.3 V was applied to the gate with 0.1 M NaCl/agarose gel. t corresponds to 63% of charge of the equivalent RC circuit modeling the ENODe ionic circuit, when a square voltage pulse was applied at the gate electrode (N = 3).
Download figure:
Standard image High-resolution imageIn detail, the mobility of ionic species within OECTs is representable using an equivalent ionic RC circuit, connecting a resistor and a capacitor in series. Consequently, the electrochemical processes involved in doping/de-doping the PEDOT:PSS channel can be described as the charging and discharging phases of this equivalent RC circuit, distinguished by a time constant (τ) [36]. This parameter indicates not only the duration required to charge the equivalent circuit from a discharged state to 63.2% of its maximum charge but also serves as a metric for evaluating the electrochemical dynamics. For the glycerol-based electrolyte, the OECT time constant (τ) increased non-linearly with the increasing ratio between glycerol and water independently of the device type (figure 2(c)). This suggests that the network of water surrounding glycerol inside the electrolyte was impacted by the formation of hydrogen bonds between molecules, and that these bonds had a significant effect on hydrated ions' migration when the electrolyte contained more glycerol than water [30], as depicted in figure 1(a). In contrast, spin coated devices displayed a time constant comparable to the liquid electrolyte case when 0.6% agarose gel electrolyte was present (figure 2(d)). There was a slight increase of time constant with agarose gel in the case of printed devices, but this variation (from 0.25 s to 0.4 s) was still negligible compared to that reported for the highest glycerol percentage. This is because the pore sizes [37] in the agarose hydrogel does not affect the cation migration during the ionic-electronic signal transduction.
To further evaluate the neuromorphic behavior of ENODes, the transistor current was measured when a transient gate modulation (with corresponding channel de-doping)[16] occurred upon the application of Vgs input consisting of six subsequent square pulses at 0.3 V for 3 s duration (individual pulse ON time) and a 9 s delay between pulses.
When agarose gel was used as electrolyte, the amplitude of the pulse and the short-term modulation of the channel current was similar to the aqueous electrolyte case (figures 3(a) and (b)). The measurements resulting from the same stimulation performed with different concentrations of glycerol are reported in figure S3. By integrating the gate current occurring during the application of the gate voltage pulse (figure 3(c)), the charge injected by the gate were calculated (figure 3(d)). Then, the retained charge was obtained by integrating the gate current with the removal of the pulse and subtracting this contribute from the charge injected [38] (figure 3(e)). A larger amount of injected and retained charges was observed for printed ENODes (higher thickness) compared to spin coated ones, both with liquid and tissue-like agarose gel electrolytes [39]. For both conditions, the retained charge gives indication about the ionic equilibrium reached after the stimulation through pulses.
Figure 3. Short term plasticity with agarose gel. (a), Channel current (with respect to the initial value I0) of printed ENODes when 6 voltage pulses of 0.3 V were applied to the gate with 0.1 M NaCl in water and 0.1 M NaCl in agarose gel. (b), Channel current (with respect to the initial value I0) of spin coated ENODes when six voltage pulses of 0.3 V were applied to the gate with 0.1 M NaCl in water and 0.1 M NaCl/agarose gel. (c), Gate current and voltage of spin coated ENODes with 0.1 M NaCl in water and 0.1 M NaCl/agarose gel. (d), Charge injected at the gate of spin coated and printed ENODes with 0.1 M NaCl in water and 0.1 M NaCl in agarose gel. (N = 3) (e), Charge retained at the channel of spin coated and printed ENODes with 0.1 M NaCl in water and 0.1 M NaCl in agarose gel. (f), Channel current of spin coated ENODes when six voltage pulses of 0.3 V were applied using three different delays, corresponding to multiples (1, 5 and 10) of the time constant that are equal to (400 ms, 2 s and 4 s) (N = 3). (g), Percentage conductance variation of the channel current when different pulses delays were used. This variation indicates the degree of paired pulse facilitation (PPF, N = 3).
Download figure:
Standard image High-resolution imageAs example of biologically plausible pattern of short-term memory, the PPF was replicated in the presence of the agarose gel electrolyte. At this purpose, trains of voltage pulses with the same amplitude and 6 s ON time were applied at the gate of ENODes by varying the delay between two subsequent pulses as multiples of the time constant [38] such as 1τ, 5τ and 10τ. Resulting PPF values for spin coated devices are reported in figure 3(f) (printed devices in figure S4). The first and last point were considered for calculating the percentage of the residual channel conductance modulation after the stimulation at different delays. A larger plastic modulation was found for the shorter delay (400 ms, figure 3(g)) whereas higher delays between pulses allowed injected cations to return to the gel electrolyte, resulting in a decrease of the residual channel modulation.
Printed ENODes exhibited higher PPF especially for lower time delays (figure S4) following the time response behavior (figure 2(d)), finally providing enhanced memory capability.
Lastly, long term plasticity of ENODes was investigated, considering the effect of catecholamine neurotransmitters (dopamine and serotonin) present in the tissue-like agarose electrolyte. Preliminary cyclic voltammetry measurements were carried out at the gate-electrolyte interface to define the characteristic oxidation potential of the neurotransmitters in liquid (0.1 M NaCl) and agarose-based electrolytes (figure S5).
Previous works showed that PEDOT:PSS can support oxidation reaction of neurotransmitters in liquid electrolytes featuring characteristic potentials at 0.2–0.4 V [40]. Gate electrodes processed from a blend of PEDOT:PSS ethylene glycol, GOPS and DBSA (see materials and methods) showed oxidation characteristic peaks at 0.3 V in 0.1 NaCl with 30 µM dopamine. A similar oxidation peak was detected in the electrolyte with 25% glycerol, while increasing concentrations of glycerol would lead to a peak shift (75% glycerol) or undetectable oxidation reaction (98% glycerol) probably because of a reduced diffusivity of cations in glycerol-concentrated mixture (S5(a)). In contrast, the oxidation peak was present when the dopamine solution was injected in the agarose gel (S5(b)), for the same values reported in literature [18].
Conversely, cyclic voltammetry measurements employing printed PEDOT:PSS SV3 electrodes did not exhibit any characteristic oxidation peak in in 0.1 NaCl with 30 µM dopamine. Here, an oxidation peak at 0.5 V was detected only in liquid electrolytes with higher dopamine concentration (100 µM, S5(c)) which would be not suitable for neural interfacing applications [18]. This could depend on the composition of PEDOT:PSS SV3 that contains different additives (not specified by the vendor) compared to the blend of PEDOT:PSS PH1000. Moreover, the thickness of the printed layers is higher than that of the spin coated ones, and this could lead to higher gate capacitance. Thus, the oxidation current of 30 µM dopamine may be relatively negligible compared to that of the printed PEDOT:PSS.
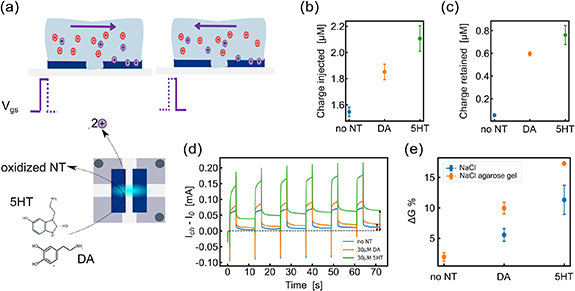
Therefore, long-term potentiation of large spin coated ENODes was further investigated considering neurotransmitters (30 µm dopamine and 30 µm serotonin) in the tissue-like agarose-gel electrolyte to exploit the contribution of additional protons produced during the oxidation reaction at the gate electrode (figure 4(a)). As stimulation signal, trains of six 0.3 V squared pulses with a 3 s ON time and a 9 s delay were applied to the gate terminal of devices. The redox reaction allowed for increasing charge injected by the gate (figure 4(b)) compared to the case when neurotransmitters were unavailable. In addition, an increase of the charge retained at the channel after the removal of the gate pulse voltage occurred, suggesting an endured de-doping of the CP even after stimulation (figure 4(c)). In general, the presence of serotonin lead to higher injected and retained charges compared to dopamine which could be attributed to a larger number of serotonin molecules to be oxidized at the gate electrode due to their higher diffusion coefficient within the electrolyte [29]. This was also confirmed by the monitoring of the channel current modulation upon gate stimulation in the gel electrolyte with neurotransmitter inside (figure 4(d)). The conductance modulation (percentage) was calculated by considering the channel conductance values before and after the application of the gate voltage stimulation, suggesting a higher conductance variation in the presence of both neurotransmitters. In the tissue-like electrolyte with serotonin, the conductance variation was the highest (figure 4(e)).
Figure 4. Neurotransmitter/gel-mediated long-term plasticity in large devices. (a), Schematics of neurotransmitter oxidation and PEDOT:PSS reduction. (b), Charge injected at the gate of spin coated ENODes with 0.1 M NaCl in agarose gel and the addition of 30 µM dopamine and 30 µM serotonin (N = 3). (c), Charge retained at the channel of spin coated ENODes with 0.1 M NaCl in agarose gel with the addition of 30 µM dopamine and 30 µM serotonin (N = 3). (d), Channel current (with respect to the initial value I0) of spin coated ENODes with agarose gel electrolyte when trains of voltage pulses of 0.3 V were applied to the gate with 0.1 M NaCl electrolyte and the addition of 30 µM dopamine and 30 µM serotonin. (e), Conductance variation percentage of the spin coated ENODe with agarose gel electrolyte with and without the addition of 30 µM dopamine and 30 µM serotonin (N = 3).
Download figure:
Standard image High-resolution image4. Conclusions and outlook
Here, we showed the effect on short- and long- term plasticity of planar ENODes using PEDOT:PSS blend materials for the channel and gate electrodes and interfacing with liquid and tissue-like electrolytes.
By comparing the figures of merit of the OECTs, including transconductance and ON–OFF ratio, printed devices demonstrated higher amplification capabilities compared to larger PEDOT:PSS spin coated devices. When considering the transient behavior of the device, similar time responses were obtained for all device's types. We observed that the electrolyte composition played a major role: indeed, with increasing concentration of glycerol inside glycerol/water mixed electrolyte, time constant increases in a non-linear behavior. On the contrary, the agarose gel electrolyte polymeric structure rather favored the diffusivity of ions, thus supporting a comparable transient behavior in all investigated devices.
When the long-term plasticity based on neurotransmitter oxidation was considered, larger spin coated ENODes featured high conductance modulation upon subsequent pulses voltage application at the gate electrode to oxidize dopamine and serotonin molecules both in liquid and in tissue-like agarose gel electrolytes. While the composition of the gate electrode of printed ENODes did not support an effective oxidation of neurotransmitters. Moreover, tissue-like agarose gel electrolyte can be adopted for ENODes to support short-term and long-term plasticity.
Acknowledgments
F S. and D R acknowledge funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (BRAIN-ACT, Grant Agreement No. 949478).
F S, D R and F C acknowledge funding from Globalink Research Award from Mitacs and Kathel Cindy Dongnang Ngoula to be part of the exchange program.
The authors acknowledge contribution from Floriane Miquet-Westphal, Felix Reul, Jacopo Di Russo and Marco Buzio for rheological measurements.
Data availability statement
The data cannot be made publicly available upon publication because no suitable repository exists for hosting data in this field of study. The data that support the findings of this study are available upon reasonable request from the authors.
Author contributions
The authors declare no competing interests.
Supplementary data (1.5 MB DOCX)