Abstract
-
Background
Diseases affecting the lungs and airways contribute significantly to the global burden of disease. The problem in low- and middle-income countries appears to be exacerbated by a shift in global manufacturing base to these countries and inadequate enforcement of environmental and safety standards. In Ghana, the potential adverse effects on respiratory function associated with occupational wood dust exposure have not been thoroughly investigated.
-
Methods
Sixty-four male sawmill workers and 64 non-woodworkers participated in this study. The concentration of wood dust exposure, prevalence and likelihood of association of respiratory symptoms with wood dust exposure and changes in pulmonary function test (PFT) parameters in association with wood dust exposure were determined from dust concentration measurements, symptoms questionnaire and lung function test parameters.
-
Results
Sawmill workers were exposed to inhalable dust concentration of 3.09 ± 0.04 mg/m3 but did not use respirators and engaged in personal grooming habits that are known to increase dust inhalation. The sawmill operators also showed higher prevalence and likelihoods of association with respiratory symptoms, a significant cross-shift decline in some PFT parameters and a shift towards a restrictive pattern of lung dysfunction by end of daily shift. The before-shift PFT parameters of woodworkers were comparable to those of non-woodworkers, indicating a lack of chronic effects of wood dust exposure.
-
Conclusions
Wood dust exposure at the study site was associated with acute respiratory symptoms and acute changes in some PFT parameters. This calls for institution and enforcement of workplace and environmental safety policies to minimise exposure at sawmill operating sites, and ultimately, decrease the burden of respiratory diseases.
-
Keywords: Lung function; Wood workers; Wood dust; FVC; FEV1
BACKGROUND
Chronic diseases affecting the lungs and airways, including chronic obstructive pulmonary disease (COPD), asthma, occupational lung diseases and lung cancer constitute a significant global health burden.
1 This burden reflects significantly in the potential years of productive life lost due to morbidity and mortality associated with these diseases, or the disability-adjusted life-years (DALYs). In 2019, COPD and lung cancer were ranked among the ten foremost causes of DALYs in males older than 50 years.
1 Asthma affected an estimated 339 million people globally in 2016 and contributed 23.7 million DALYs, making it the 28th leading cause of global disease burden.
2 Worryingly, the burden of chronic respiratory diseases in middle- and low-income countries is high,
1,3,4 posing additional difficulties for the already fragile health systems, which in turn exacerbates disease burden, in a vicious cycle.
5
Occupational exposure to wood dust is recognized as an important driver of chronic respiratory diseases globally. However, it is of greater concern in developing countries due to increasing proportion of global manufacturing base and lax enforcement of workplace safety regulations.
6,7 The wood processing industry is a significant source of wood dust, as all stages of wood processing including peeling, slicing, sawing, routing, sanding and planing, produce dust. Hardwoods, such as those that predominate in tropical countries, produce greater quantities of dust because of greater densities of wood cells than softwoods found in more temperate climates.
8 Hardwoods commonly processed in Ghana, West Africa, include obeche (wawa), iroko (odum) and mahogany, which have densities between 390 and 660 kg/m
3.
9
Dust exposure at wood processing sites could be minimised by the use of effective ventilation systems, and respiratory protective equipment (RPEs) such as respirators, as well as effective disposal of sawdust.
10 However, ventilators and RPEs may be absent and sawdust may be discarded on site,
11 which could expose sawmill workers, carpenters, cleaning and maintenance staff, construction workers and ship or boat builders to high levels of wood dust.
8
The wood industry contributes significantly to Ghana’s economy, placing fourth as the country’s highest earner and providing direct employment to more than 100,000 people.
12 Regardless, workplace conditions of wood workers that could expose them to the potential harmful effects of wood dust have received little attention. A growing body of evidence, including those from studies carried out in Nigeria—a country in West Africa with similar wood types, industrial wood processing techniques and workplace conditions—indicates that high wood dust exposure is associated with increased frequency of respiratory symptoms and impaired lung function, as reflected in significant decrease in selected respiratory parameters such as the one-second forced expiratory volume (FEV
1) and forced vital capacity (FVC).
13,14,15,16,17,18
The present study addresses the effect of wood dust exposure on respiratory function of the Ghanaian woodworker by examining the wood dust exposure at some sawmill operating sites in the Cape Coast Metropolis, the prevalence of respiratory symptoms and occurrence of acute and chronic changes in respiratory function compared to healthy controls.
METHODS
Study design and sample size
This was a cross-sectional study conducted over a period of 10 months between September 2019 and July 2020. The study population consisted of male sawmill workers from the 3 biggest sawmill operating sites in 3 localities (i.e., Abura, Kakumdo and Esuekyir) in the Cape Coast Metropolis of the Central Region of Ghana. The study sites were situated near major roads in unpaved, open places. The workers were involved in activities such as sawing, planing, sanding and removal of saw dust from the saw mill. All consenting male woodworkers at each study site were recruited into the study but only those who provided complete responses to the questionnaire were included in the analysis. Compartmentalisation of space or function was little or absent, as all workers engaged in similar activities within the same open space. Administrative staff were not included in the sampling. The reference group of 64 male subjects was selected by convenience sampling from among office workers, security personnel and students of the University of Cape Coast. Student participants were asked to quantify their daily workload from hours spent in lecture rooms, laboratory and library. The academic year of the student was used in lieu of work experience.
Dust sampling
Dust exposure levels were measured at Abura and Kakumdo sawmill operating sites. The inhalable concentration collected during one work shift (lasting approximately 8 hours) was measured with a personal dust sampler (AirChek XR5000 pumps; SKC, Blandford Forum,UK) equipped with dust filters assembled on a worker’s chest within their breathing zone. The airflow was set to 3.5 L/minute. A total of 6 samples were collected from the timber markets (3 samples each at Abura and Kakumdo). Two control samples were collected at the workplaces of the reference group—the first was collected on a security guard stationed outside an office building and the second on an indoor office worker.
Questionnaire survey
A structured questionnaire was used to collect anthropometric data of participants (age, height, sex and weight), medical history, work experience and daily work hours, personal grooming habits and data on status of respiratory symptoms during vacation (off work periods). The questionnaire, which was based on the American Thoracic Society (ATS) questionnaire for respiratory symptoms and was adjusted to fit local conditions, obtained anthropometric characteristics of participants, presence of respiratory symptoms, smoking status, grooming habits, i.e., whether or not participants regularly shaved their nasal vibrissae and facial hair, personal and family medical history, etc. The questionnaires were administered to the participants by the researchers. This was done in the English language but was translated into the 2 predominant local languages of the area, i.e., Twi or Fante, for those who did not speak English.
Pulmonary function test (PFT)
The lung function of the participants was assessed using a computer-based, hand-held spirometer (MIR MiniSpir spirometer; A-M Systems, Carlsberg, WA, USA) connected to the WinspiroPRO 7.6 software. Spirometry was carried out with the subject sitting in an upright position. The PFT parameters FVC, FEV
1, and forced expiratory ratio (FER) were measured and saved electronically. Spirometry was performed in 2 sessions; before exposure to wood dust at the start of work for a new week (“before shift”) and after exposure to wood dust at the close of work on first day of the week (“after shift”). Before shift and after shift assessments for the control group were performed, respectively, in the morning at the beginning of work and in the afternoon shortly before close of work. The spirometry manoeuvre was done according to ATS/European Respiratory Society guidelines.
19 For each session, the manoeuvre was performed at least 3 times in each subject and the best score for each subject was selected as the final score.
Ethical clearance and approval were obtained from the University of Cape Coast Institutional Review Board with approval number UCCIRB/EXT/2019/25. Informed consent was also obtained from all participants in this study. Confidentiality in the management of participants’ information was ensured. Prospective participants were informed of their liberty to withdraw from the study at any time, if they so wished.
Comparisons
Differences in wood dust exposure between the 2 groups were assessed by comparing the concentration of inhalable dust. Due to the location of sawmills in open sheds along major roads, the potential contribution of inorganic dust particles blown from the road and bare floors was examined by expressing inhalable dust as organic and inorganic fractions. To determine association between wood dust exposure status of the subjects and anthropometric parameters, smoking status, work hours and experience, and personal grooming habits, the respective measures for the study population and reference group were compared. The potential adverse effects of wood dust on pulmonary function were assessed by comparing the likelihoods of association of the respiratory symptoms as well as changes in PFT parameters between woodworkers and the reference population. To separate acute and chronic effects of wood dust exposure, the observed versus expected disappearance or improvement of respiratory symptoms among woodworkers during vacations or weekends were compared. Furthermore, cross-shift changes in PFT among wood workers were considered “acute effects” while differences in PFT between wood workers and controls occurring within the same shift were considered “chronic effects.”
To examine factors that may exacerbate wood dust exposure among wood workers, the responses to perceived workload stress (a self-report of work stress or tiredness and number of hours applied at work), dust exposure insight (self-knowledge of severity of exposure to dust) and use of respirators as protective gear against dust inhalation were assessed. A combination of high workload stress, high exposure insight but low use of respirators was considered exacerbating for adverse wood dust effects.
Statistics
Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 26 (IBM Corp., Armonk, NY, USA). The data was organised and analysed by using descriptive and inferential statistics. Descriptive statistics was used to present anthropometric and other variables by frequency, mean, standard mean error and percentage. Statistical comparisons of wood worker and reference group variables were performed using the paired samples t-test, χ2 test and multiple linear regression, depending on the test conditions. The association between wood dust exposure and frequency of respiratory symptoms was tested using binary logistic regression. Significance was set at p-values less than 0.05.
RESULTS
A total of 128 people, consisting of 64 members each for woodworkers and a reference group of non-woodworkers, participated in this study. The woodworkers had a higher mean age (
p < 0.001), higher number of years of work (
p < 0.01), higher number of working days in a week (
p < 0.001) and higher number of hours of work per day (
p < 0.001) compared to the reference group (
Table 1). On the other hand, the mean height of the reference group was significantly taller than woodworkers (
p = 0.012). No significant differences were found between the mean weight of the groups (
p > 0.05). Although a slightly higher proportion of woodworkers smoked than the reference population (7.81% vs. 3.13%), the difference was insignificant (
p = 0.244), and therefore, any potential effects of smoking on lung function were not explored further. Also, a significantly higher proportion of woodworkers regularly shaved their nasal vibrissae (40.63% vs. 6.56%,
p < 0.001). No statistical difference was found between the proportions of subjects who regularly shaved their moustache.
Table 1 Demographic characteristics of study participants
|
Variable |
Woodworkers (n = 64) |
Reference group (n = 64) |
p-value |
|
Age (year) |
29.67 ± 10.19 |
24.05 ± 6.95 |
< 0.001a
|
|
Height (cm) |
171.48 ± 6.90 |
174.40 ± 5.97 |
0.012a
|
|
Weight (kg) |
66.25 ± 11.08 |
67.05 ± 11.77 |
0.694a
|
|
Work experience (year) |
8.53 ± 8.40 |
4.96 ± 5.35 |
0.007a
|
|
Daily work hours |
8.97 ± 1.97 |
6.61 ± 2.70 |
< 0.001a
|
|
Working days in a week |
6.00 ± 0.18 |
4.69 ± 0.55 |
< 0.001a
|
|
Smokers (%) |
7.81 |
3.13 |
0.244b
|
|
Regularly shave moustache (%) |
60.94 |
68.75 |
0.185b
|
|
Regularly shave nasal vibrissae (%) |
40.63 |
6.56 |
< 0.001b
|
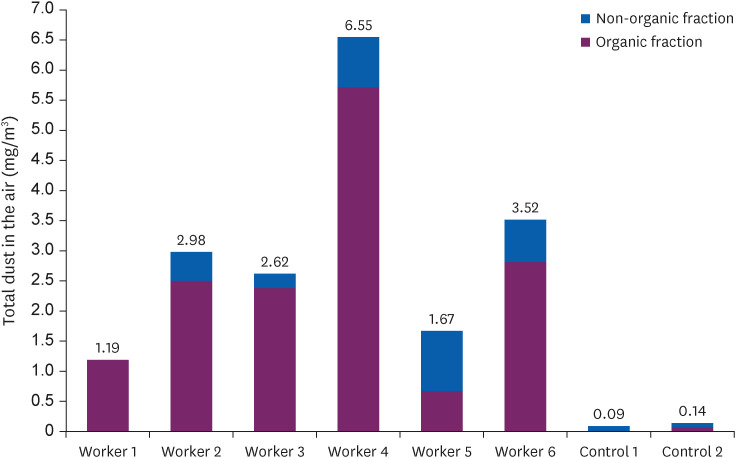
Fig. 1 shows the level of exposure to dust among woodworkers and the reference group. The mean quantity of dust exposure for 6 samples of inhalable dust measurement at 2 different timber markets was 3.09 mg/m
3 (range 1.19 to 6.55 mg/m
3). Two measurements at the workplaces of the reference population showed dust exposures of 0.09 mg/m
3 (indoor worker) and 0.14 mg/m
3 (outdoor worker). The larger fraction of inhalable dust measured at sawmill sites was organic wood dust (2.54 mg/m
3), while the inorganic dust level was 0.55 mg/m
3. The study population was exposed to significantly higher quantities of inhalable dust than the reference population (3.09 ± 0.04 mg/m
3 vs. 0.12 ± 0.03 mg/m
3,
p = 0.012), organic fraction of inhalable dust (2.54 ± 1.76 mg/m
3 vs. 0.04 ± 0.05 mg/m
3,
p = 0.017) and inorganic fraction of inhalable dust (0.55 ± 0.38 mg/m
3 vs. 0.08 ± 0.01 mg/m
3,
p = 0.030).
Fig. 1 Total levels of inhalable personal exposure (mg/m3) to organic dust (purple bars) and inorganic dust (blue bars) among wood workers and reference populations during an 8-hour work shift.
Respiratory symptoms occurred more frequently among woodworkers (
Table 2). All the respiratory symptoms examined occurred at greater frequencies and risks of association among woodworkers than the reference population, excepting “breathlessness during exercise.” Sneezes (75%), wheezes (62.5%), rhinorrhoea (62.5%), coughs (48.5%) and sputum (40.6%) were the commonest symptoms among woodworkers, occurring at 16.20 folds, 51.67 folds, 10.71 folds and 21.21 folds, respectively, more than the reference population. The woodworkers were also more likely to develop dry cough (10.65 times), fevers (14.09 times), breathlessness at rest (5.43 times), hoarseness or loss of voice (4.98 times) and dyspnoea (21.11 times).
Table 2 Frequency of respiratory symptoms among woodworkers and reference group
|
Symptom |
Wood-workers |
Reference group |
Risk ratioa (exposed/non-exposed) |
95% CI (lower–upper) |
p-valueb
|
|
Wheezing |
40 (62.5) |
2 (3.1) |
51.67 |
11.57–230.69 |
< 0.001*
|
|
Sneezing |
48 (75.0) |
10 (15.6) |
16.20 |
6.72–39.08 |
< 0.001*
|
|
Rhinorhoea |
40 (62.5) |
7 (13.5) |
10.71 |
4.17–27.51 |
< 0.001*
|
|
Cough |
31 (48.4) |
4 (6.3) |
14.09 |
4.58–43.39 |
< 0.001*
|
|
Dry cough |
22 (34.4) |
3 (4.7) |
10.65 |
3.00–39.88 |
< 0.001*
|
|
Fevers |
31 (48.8) |
4 (6.3) |
14.09 |
4.58–43.39 |
< 0.001*
|
|
Sputum |
26 (40.6) |
2 (3.1) |
21.21 |
4.76–94.47 |
< 0.001*
|
|
Breathlessness at rest |
17 (26.6) |
4 (6.3) |
5.43 |
1.71–17.21 |
0.002*
|
|
Breathlessness during exercise |
19 (29.7) |
22 (34.4) |
0.86 |
0.38–1.70 |
0.570 |
|
Hoarseness/Loss of voice |
19 (29.7) |
5 (7.8) |
4.98 |
1.73–14.36 |
0.002*
|
|
Personal history of asthma |
1 (1.6) |
3 (4.7) |
0.32 |
0.03–3.19 |
0.310 |
|
Dyspnoea |
19 (29.7) |
1 (2.0) |
21.11 |
2.72–164.12 |
< 0.001*
|
Table 3 presents exposure profiles, RPE use and chronicity of respiratory symptoms among wood workers. A larger proportion of woodworkers reported high workload stress (98.4%) and high dust exposure insight (98.4%). However, only 3 respondents representing 4.7% of woodworkers, regularly used RPEs, while 61 respondents, representing 95.3% did not use them, citing discomfort associated with wearing RPEs (14 respondents, representing 22.3%) and non-availability of RPEs due to cost (46 respondents, representing 73.0%). A few woodworkers (3 respondents, representing 4.7%) had no reason for working without RPEs. This shows a combination of high daily work exposure to wood dust among woodworkers but little protection from the exposure.
Table 3 Exposure profiles, respiratory protective equipment and chronicity of respiratory symptoms
|
Variable |
Woodworkers |
|
Perceived workload stress |
|
|
High |
63 (98.44) |
|
Low |
1 (1.56) |
|
Dust exposure insight |
|
|
High |
63 (98.44) |
|
Low |
1 (1.56) |
|
Nose mask use at workplace |
|
|
Yes |
3 (4.69) |
|
No |
61 (95.31) |
|
Reason for non-use of mask |
|
|
Discomfort/difficulty breathing |
14 (22.22) |
|
Non availability/cost |
46 (73.02) |
|
No reason |
3 (4.76) |
|
Improve on non-working days |
|
|
Yes |
43 (67.19) |
|
No |
21 (32.81) |
|
Disappear on non-working days |
|
|
Yes |
37 (57.81) |
|
No |
27 (42.19) |
|
Improve during vacations |
|
|
Yes |
43 (67.19) |
|
No |
21 (32.81) |
|
Disappear during vacations |
|
|
Yes |
37 (57.81) |
|
No |
27 (42.19) |
|
Symptom improvement latency during vacation (days) |
|
|
1 |
28 (43.75) |
|
2 |
9 (14.06) |
|
3 |
15 (23.44) |
|
Other day in first week |
6 (9.38) |
|
No answer |
6 (9.38) |
On chronicity of symptoms, a higher proportion of woodworkers (43 respondents, representing 67.19%) reported having less severe respiratory symptoms on non-working days than those who found no improvement in symptoms (21 respondents, representing 32.81%). A higher proportion of woodworkers (37 respondents, representing 57.81%) also stated complete disappearance of respiratory symptoms on off-work days than those who reported persistence of those symptoms (27 respondents, representing 42.19%). Among woodworkers who reported improvement or disappearance on non-working days, 28 persons, representing 43.75% reported improvement on the first day of vacation, 9 respondents, representing 14.06% on second day, 15 respondents, representing 23.44% on third day and 6 respondents, representing 9.38% on other days. A further 6 respondents, representing 9.4% provided no answer. Together, the results on symptoms chronicity show that respiratory symptoms among a majority of woodworkers (81.25%) improved quickly during off work periods, whereas 18.75% of woodworkers experienced chronic respiratory symptoms during the off-work period.
The results of the PFT are shown in
Table 4 for a total of 49 woodworkers and 64 members of the reference group who competed before and after shift spirometries. Acute effects of wood dust exposure on PFT parameters were assessed by comparing cross-shift changes in PFT scores of the woodworkers, i.e., differences between scores obtained at the start of shift (before shift) and at the end of shift (after shift). Significant cross-shift differences were obtained for FVC (3.62 ± 0.11 vs. 3.49 ± 0.10,
p = 0.014), and for FEV
1 (3.11 ± 0.08 vs. 3.00 ± 0.08,
p = 0.021) but not FER (86.88 ± 1.34 vs. 86.61 ± 1.31,
p = 0.697). Assessment of chronic effects of wood dust exposure was performed by comparing PFT parameters of woodworkers and the reference population obtained within the same shift. Owing to differences in several anthropometric and sociodemographic parameters between woodworkers and the reference, the effect of each parameter on PFT scores was examined.
Table 5 shows that wood dust exposure was not significantly associated with FVC (
p = 0.202), FEV
1 (
p = 0.411) and FER (
p = 0.558) scores. Rather, differences in height, weight and age between woodworkers and reference group accounted for PFT differences measured within the same shift.
Table 4 Cross-shift comparison of spirometry scores of wood workers
|
Variable |
Before shift |
After shift |
p-valuea
|
|
FVC (L) |
3.62 ± 0.11 |
3.49 ± 0.10 |
0.014*
|
|
FEV1 (L) |
3.11 ± 0.08 |
3.00 ± 0.08 |
0.021*
|
|
FER (FEV1/FVC%) |
86.88 ± 1.34 |
86.61 ± 1.31 |
0.697 |
Table 5 Association between anthropometric parameters and dust exposure status on PFT
|
Variable |
PFT parameter |
β (95% CI)a
|
p-valueb
|
|
Wood dust exposure status |
FVC |
0.142 (−0.08, 0.36) |
0.202 |
|
FEV1
|
−0.078 (−0.27, 0.12) |
0.411 |
|
FEV1/FVC |
1.279 (−3.03, 5.59) |
0.558 |
|
Height |
FVC |
0.038 (0.02, 0.06) |
< 0.001*
|
|
FEV1
|
0.033 (0.02, 0.05) |
< 0.001*
|
|
FEV1/FVC |
0.137 (−0.22, 0.50) |
0.452 |
|
Weight |
FVC |
0.06 (−0.01, 0.02) |
0.246 |
|
FEV1
|
0.003 (−0.01, 0.01) |
0.514 |
|
FEV1/FVC |
−0.231 (−0.44, −0.03) |
0.027*
|
|
Age |
FVC |
−0.015 (−0.03, 0.00) |
0.044*
|
|
FEV1
|
−0.014 (−0.03, 0.00) |
0.024*
|
|
FEV1/FVC |
−0.189 (−0.471, 0.09) |
0.188 |
Together, the spirometry results presented in
Tables 4 and
5 indicate that whereas wood dust exposure is associated with significant acute changes in some PFT parameters, no chronic changes associated with wood dust exposure alone was found.
The effect of wood dust exposure on respiratory status was assessed by comparing the respiratory status of 48 woodworkers and 63 reference subjects, who strictly completed before- and after-shift spirometries on the same day. The lung status of each was subject was classified by the WinspiroPRO software as normal (FER, FVC and FEV
1 all within normal of predicted), obstructive (FER and FEV
1 < normal of predicted) or restrictive (FVC and FEV
1 < normal of predicted accompanied by FER > normal) pattern, in line with previously established criteria.
20 In the before-shift period, 49 participants within the reference population, representing 77.8% had normal respiratory status, 13 participants, representing 20.6% had a restrictive lung status and 1 participant had an obstructive status. During the same period, 35 woodworkers, representing 72.9% had normal respiratory status, 11 participants, representing 22.9% had restrictive lung condition and 2 participants, representing 4.2% had an obstructive lung status. This distribution of respiratory status was not statistically significant (
p = 0.663). In the after-shift period, 51 (81%) participants in the reference group examined had normal respiratory status while 12 (19%) had a restrictive status. However, the number of woodworkers with normal respiratory status had reduced to 26 (54.2%), the number with restrictive lung status had increased to 21 (43.8%) and only 1 participant (2.1%) had an obstructive status, showing a significant shift in the respiratory status of woodworkers towards a restrictive pattern by the end of a daily shift (
p = 0.008).
DISCUSSION
Significant differences in various anthropometric and demographic indices were found between woodworkers and the reference group, including age, height, number of years in active employment, number of work days in a week and the number of daily work hours. Some of these differences may confound absolute PFT scores, e.g., age and height,
21,22 and so the effects of exposure on PFT parameters were studied after controlling for some of these differences. Furthermore, the number of smokers among both study and reference groups was low and the difference was not significant, showing that smoking was not a confounding factor. In contrast with another study in Iran that found that as much as 41% of the study population and 38% of the reference population smoked,
15 there is a low prevalence of smoking in Ghana,
23,24 which explains this finding.
Interestingly, a significantly higher proportion of woodworkers regularly shaved their nasal vibrissae than the reference group (40.63% vs. 6.56%). Because the nasal vibrissae trap large particles from entering the nasal passages and improve the efficiency of the passages in the long-term,
25 shaving them may worsen the impact of wood dust exposure. However, trimming the nasal vibrissae may relieve nasal obstruction,
26 and because wood dust exposure reduces nasal patency,
27 this grooming behaviour may serve this purpose among woodworkers. Nasal trimming may also be common among wood workers due to cosmetic reasons, since the vibrissae become more noticeable when they trap particles in dusty environments.
In this study, woodworkers were exposed to a wood dust concentration of 3.09 mg/m
3, compared with exposure levels of 0.115 mg/m
3 for non-woodworkers. Such high exposures are expected at sawmill operating sites, where various wood processing activities produce dust. Additionally, wood wastes generated by the timber industry in Ghana is not properly disposed and is often discarded on site,
11 which contributes to high exposure. Consequently, the exposure of woodworkers exceeded the threshold limit value-time weighted average (TLV-TWA) of 1 mg/m
3 per 8 hours for hard woods.
28 A higher exposure to inorganic dust as a fraction of inhalable dust at sawmill operating sites is expected on account of the general location of the sites by major roads in open, unpaved sheds. Exposure to inorganic dust particles is associated with increased frequency of respiratory symptoms and long-term adverse effects on respiratory function.
29,30
This study has found a high frequency of respiratory symptoms among woodworkers, as well as high likelihoods of association between wood dust exposure and presence of respiratory symptoms (
Table 2). This finding is in agreement with previous reports.
14,15,16,17,18,31,32,33,34,35,36,37 The allergenic effects of wood dust have been firmly established in numerous studies,
38,39,40,41,42,43,44,45,46 which explains the association, although a few other studies have reported finding no adverse respiratory symptoms with wood dust exposure.
35,36,47 Some of these differences may arise from differences in wood type, use of RPEs and adherence to workplace safety protocols, grooming behaviour and other confounding demographic, genetic, anthropometric and behavioural parameters. For instance, the study population of the present study did not wear respirators, in spite of gruelling work hours and awareness of high wood dust exposure, either because cost-cutting measures instituted by supervisors and employers limited their provision or the workers reported discomfort associated with wearing them (
Table 3). The real reason RPEs were not used by the workers may well be that they were oblivious of the potential injurious consequences of such exposure, perhaps, due to the low educational levels and lack of opportunity among Ghanaian artisans.
A few studies have reported increased obstructive lung dysfunction among woodworkers compared to controls.
34,39 On the other hand, several other previous studies have reported little evidence of increased obstructive lung dysfunction in association with wood dust exposure. Rather, restrictive lung condition appears to be a more common pattern among subjects exposed to both soft and hardwoods,
15,16,48 which is supported by reports of increased inflammation and lung fibrosis associated with wood dust exposure.
49,50 Indeed, in the present study a higher proportion of restrictive lung dysfunction (52%) was found among the woodworkers after exposure to wood dust (
Table 6), which supports the above.
Table 6 Respiratory status before start of shift and end of shift among woodworkers
|
Shift |
Variable |
Normal |
Restrictive |
Obstructive |
χ2 statistic |
p-valuea
|
|
Before shift |
Reference |
49 (77.8) |
13 (20.6) |
1 (1.6) |
0.821 |
0.663 |
|
Woodworkers |
35 (72.9) |
11 (22.9) |
2 (4.2) |
|
After shift |
Reference |
51 (81) |
12 (19) |
0 (0) |
9.722 |
0.008*
|
|
Woodworkers |
26 (54.2) |
21 (43.8) |
1 (2.1) |
On the question of whether wood dust exposure produces effects on PFT parameters, this study found a significant cross shift decline in the scores of the FVC and FEV
1 but not FER among woodworkers (
Table 4). This finding suggests an acute decrease of respiratory function that is consistent with the reported acute allergenic effects of wood dust exposure.
38,39,40,41,42,43,44,45,46 However, the absence of a chronic effect of wood dust exposure when confounding factors were controlled (
Table 5), suggests that the between-shifts changes in PFT scores of woodworkers in the previous shift were, in the time interval of about 12 hours between shifts, completely reversible.
An explanation for the cross-shift decline in some PFT parameters among woodworkers, especially when interpreted with the absence of a chronic effect of wood dust exposure, could be reduced expiratory effort during spirometry by tired woodworkers at the end of a long workshift. However, reference subjects did not show this shift. Moreover, some studies have reported a shift towards increased leucocyte recruitment to the lungs, increased cytokine, chemokine and chemokine receptor expression, along with a restrictive lung status upon wood dust exposure.
15,51 Due to various logistical and technical limitations, including a lack of documentation at the sawmill sites, an assessment of wood type, wood treatment with preservatives, harmful microbial contamination and other factors that could clarify these findings was not performed. Further research to explore causality and underlying mechanisms of allergenic effects to acute wood dust exposure in the study area is required.
The absence of chronic changes in PFT scores among woodworkers was unexpected but appears to be supported by a few other studies.
35,36,47 However, some other studies reported significant chronic effects.
15,16,31,32,33,37 The difference in outcome may be due to wood type and amount of exposure, microbial and chemical contaminants in the work environment, number of years of exposure and other sociodemographic and anthropometric variables. One study,
15 for example, found chronic differences in PFT among woodworkers in Iran compared with unexposed controls but the concentration of dust in air in that study was 6.76 ± 1.71 mg/m
3, compared with (3.09 ± 1.90 mg/m
3) found in this study. Additionally, the wood workers in that study had a mean work experience of 13.6 ± 9.91 years, compared with mean work experience of 8.53 ± 8.40 found in this study. Indeed, significant correlations between adverse pulmonary health status and intensity of wood dust exposure
52 and work experience as a wood worker
17 have been previously reported. Additionally, it must be supposed that acute inflammatory responses induced by wood dust exposure during a shift
51 resolve by the beginning of the next shift, and differences in lung status could disappear. In this respect, wood-dust exposure effects are similar to those caused by reversible byssinosis, whereby cross-shift differences in lung function emerge upon acute exposure on work days but not when workers stayed away from exposure.
53
Detailed clinical assessment of respiratory health of woodworkers from the symptoms questionnaire and results of PFT was not part of the scope of this work. A detailed examination of this question is planned in future studies. Another major limitation of the present study is failure to measure dust exposure for each wood worker, and consequently, a failure to perform a dose-response analysis between intensity of dust exposure and spirometry changes, as well as occurrence of respiratory symptoms. In the context of this study, however, all the steps of wood processing at the timber markets occur within the same space. Hence, a similar level of exposure was expected for all individuals at any given site. Nonetheless, future studies will explore this dose-response relationship in the study area.
In sum, this study has found wood dust exposure levels exceeding the TLV-TWA for this dust, as well as associated respiratory symptoms and changes in PFT parameters among the woodworkers. Although the observed effects of the exposure were mostly acute, it is to be expected that repeated cycles of acute lung injury may culminate in long-term pulmonary dysfunction and cancers, as reported in other studies.
15,38,39,42,48,54,55 This calls for immediate enforcement of environmental and personal safety protocols at sawmill operating sites. Improved ventilation, safe collection and disposal of sawdust, wearing of RPEs and education of woodworkers on the potential harmful effects of wood dust exposure are important steps towards minimising wood dust exposure, and ultimately, decreasing the burden of respiratory diseases.
CONCLUSIONS
Our findings indicate that exposure to wood dust impaired the lung function of wood workers. It is therefore imperative for stakeholders of the wood industries to put in place and enforce safety measures at the work place to minimize the negative effects of wood dust on workers and enhance their safety at the work place.
Acknowledgements
We wish to show gratitude to the executive members, owners and wood workers at Abura, Esuekyir, Kakumdo sawmill companies and control persons at University of Cape Coast for participating in the project. We also thank Statistician Ing-Liss Bryngelsson for excellent help with the data processing.
-
Funding: We gratefully acknowledge external funding from Carl Erik Levins Stiftelse Foundation from Sweden for this research.
-
Competing interests: The authors declare that they have no competing interests.
-
Authors contributions:
Conceptualization: Djankpa FT, Ekman J.
Data curation: Djankpa FT, Ekman J, Quartey P, Wiafe GA.
Formal analysis: Ussif AM, Egbenya DL, Ekman J, Quartey P.
Investigation: Ekman J, Quartey P, Ussif AM, Ricklund N, Egbenya DL, Wiafe GA, Tsegah KM, Karikari A, Löfstedt H, Djankpa FT.
Methodology: Djankpa FT, Ekman J, Quartey P, Wiafe GA, Tsegah KM, Karikari A, Löfstedt H, Ricklund N.
Writing - original draft: Djankpa FT, Ekman J, Quartey P, Ussif AM, Ricklund N, Egbenya DL, Wiafe GA, Tsegah KM, Karikari A, Löfstedt H.
Writing - review & editing: Djankpa FT, Ekman J, Ussif AM, Egbenya DL, Ricklund N, Löfstedt H, Karikari A.
References
- 1. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020;396(10258):1204–1222. 33069326.PubMedPMC
- 2. Global Asthma Network. The global asthma report. Updated 2018]. Accessed October 16, 2020].
http://www.globalasthmareport.org/
.
- 3. Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc 2014;11(3):404–406. 24673696.ArticlePubMed
- 4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3(11):e442–e442. 17132052.ArticlePubMedPMC
- 5. de-Graft Aikins A, Unwin N, Agyemang C, Allotey P, Campbell C, Arhinful D. Tackling Africa’s chronic disease burden: from the local to the global. Global Health 2010;6(1):5. 20403167.ArticlePubMedPMC
- 6. Schluger NW, Koppaka R. Lung disease in a global context. A call for public health action. Ann Am Thorac Soc 2014;11(3):407–416. 24673697.ArticlePubMed
- 7. Eyiah AK, Kheni NA, Quartey PD. An assessment of occupational health and safety regulations in Ghana: a study of the construction industry. J Build Constr Plan Res 2019;07(02):11–31.ArticlePDF
- 8. IARC Working Group on Evaluation of Carcinogenic Risks to Humans. Wood Dust and Formaldehyde. Vol. 62. Lyon, France: International Agency for Research on Cancer; 1995.
- 9. Yeboah D, Burton AJ, Storer AJ, Opuni-Frimpong E. Variation in wood density and carbon content of tropical plantation tree species from Ghana. New For 2014;45(1):35–52.ArticlePDF
- 10. Black N, Dilworth M, Summers N. Occupational exposure to wood dust in the British woodworking industry in 1999/2000. Ann Occup Hyg 2007;51(3):249–260. 17369618.PubMed
- 11. Asamoah O, Kuittinen S, Abrefa Danquah J, Quartey ET, Bamwesigye D, Mario Boateng C, et al. Assessing wood waste by timber industry as a contributing factor to deforestation in Ghana. Forests 2020;11(9):939.Article
- 12. Quansah F, Tandoh-Offin P. Determinants of performance in the wood industry in Ghana: an overview. Bus Econ Rev 2017;7(2):55.ArticlePDF
- 13. Dunga JA, Alkali NH, Adamu YM, Bathna S, Suleiman Y, Ukoli C, et al. FEV1, FVC, FEV1 /FVVC as predictors of rhinitis among saw mill workers in north central Nigeria. Niger J Med 2016;25(2):152–158. 29944313.ArticlePubMed
- 14. Hussain S, Mahmood N, Karadaky K, Ali A, Mohammad G, Mahmood O. Respiratory function among sawmill workers in different areas of Sulaimani city. Int J Med Sci Public Health 2016;5(2):351–355.Article
- 15. Neghab M, Jabari Z, Kargar Shouroki F. Functional disorders of the lung and symptoms of respiratory disease associated with occupational inhalation exposure to wood dust in Iran. Epidemiol Health 2018;40:e2018031. 30056642.ArticlePubMedPMC
- 16. Okwari OO, Antai AB, Owu DU, Peters EJ, Osim EE. Lung function status of workers exposed to wood dust in timber markets in Calabar, Nigeria. Afr J Med Med Sci 2005;34(2):141–145. 16749338.PubMed
- 17. Omole JO, Fabunmi AA, Akosile CO. Respiratory function of sawmill workers and their relationship to exposure time to wood dust seen in Nigeria. J Environ Occup Sci 2018;7(1):9–16.Article
- 18. Tobin EA, Ediagbonya TF, Okojie OH, Asogun DA. Occupational exposure to wood dust and respiratory health status of sawmill workers in South-South Nigeria. J Pollut Eff Control 2015;4(1):4–9.
- 19. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005;26(2):319–338. 16055882.ArticlePubMed
- 20. Haynes JM. Basic spirometry testing and interpretation for the primary care provider. Can J Respir Ther 2018;54(4.Article
- 21. Moore VC. Spirometry: step by step. Breathe 2012;8:232–240.Article
- 22. Talaminos Barroso A, Márquez Martín E, Roa Romero LM, Ortega Ruiz F. Factors affecting lung function: a review of the literature. Arch Bronconeumol (Engl Ed) 2018;54(6):327–332. 29496283.ArticlePubMed
- 23. Chung-Hall J, Craig L, Gravely S, Sansone N, Fong GT. Impact of the WHO FCTC over the first decade: a global evidence review prepared for the Impact Assessment Expert Group. Tob Control 2019;28(Suppl 2):s119–s128. 29880598.ArticlePubMedPMC
- 24. Owusu-Dabo E, Lewis S, McNeill A, Gilmore A, Britton J. Smoking uptake and prevalence in Ghana. Tob Control 2009;18(5):365–370. 19581276.ArticlePubMedPMC
- 25. Ozturk AB, Damadoglu E, Karakaya G, Kalyoncu AF. Does nasal hair (vibrissae) density affect the risk of developing asthma in patients with seasonal rhinitis? Int Arch Allergy Immunol 2011;156(1):75–80. 21447962.ArticlePubMedPDF
- 26. Stoddard DG Jr, Pallanch JF, Hamilton GS. The effect of vibrissae on subjective and objective measures of nasal obstruction. Am J Rhinol Allergy 2015;29(5):373–377. 26358350.ArticlePubMedPDF
- 27. Schlünssen V, Schaumburg I, Andersen NT, Sigsgaard T, Pedersen OF. Nasal patency is related to dust exposure in woodworkers. Occup Environ Med 2002;59(1):23–29. 11836465.ArticlePubMedPMC
- 28. The National Institute for Occupational Safety and Health (US). Wood dust. Updated 2011]. Accessed January 11, 2021].
https://www.cdc.gov/niosh/pel88/wooddust.html
.
- 29. Khan RK, Strand MA. Road dust and its effect on human health: a literature review. Epidemiol Health 2018;40:e2018013. 29642653.ArticlePubMedPMC
- 30. Habybabady RH, Sis HN, Paridokht F, Ramrudinasab F, Behmadi A, Khosravi B, et al. Effects of dust exposure on the respiratory health symptoms and pulmonary functions of street sweepers. Malays J Med Sci 2018;25(6):76–84. 30914881.ArticlePubMedPMC
- 31. Osman E, Pala K. Occupational exposure to wood dust and health effects on the respiratory system in a minor industrial estate in Bursa, Turkey. Int J Occup Med Environ Health 2009;22(1):43–50. 19342363.ArticlePubMed
- 32. Milanowski J, Góra A, Skórska C, Krysińska-Traczyk E, Mackiewicz B, Sitkowska J, et al. Work-related symptoms among furniture factory workers in Lublin region (eastern Poland). Ann Agric Environ Med 2002;9(1):99–103. 12088405.PubMed
- 33. Ige OM, Onadeko OB. Respiratory symptoms and ventilatory function of the sawmillers in Ibadan, Nigeria. Afr J Med Med Sci 2000;29(2):101–104. 11379437.PubMed
- 34. K Hosseini D, Malekshahi Nejad V, Sun H, K Hosseini H, Adeli SH, Wang T. Prevalence of respiratory symptoms and spirometric changes among non-smoker male wood workers. PLoS One 2020;15(3):e0224860. 32187180.ArticlePubMedPMC
- 35. Borm PJA, Jetten M, Hidayat S, van de Burgh N, Leunissen P, Kant I, et al. Respiratory symptoms, lung function, and nasal cellularity in Indonesian wood workers: a dose-response analysis. Occup Environ Med 2002;59(5):338–344. 11983850.ArticlePubMedPMC
- 36. Bohadana AB, Massin N, Wild P, Toamain JP, Engel S, Goutet P. Symptoms, airway responsiveness, and exposure to dust in beech and oak wood workers. Occup Environ Med 2000;57(4):268–273. 10810114.ArticlePubMedPMC
- 37. Bislimovska D, Petrovska S, Minov J. Respiratory symptoms and lung function in never-smoking male workers exposed to hardwood dust. Open Access Maced J Med Sci 2015;3(3):500–505. 27275278.ArticlePubMedPMCPDF
- 38. Alonso-Sardón M, Chamorro AJ, Hernández-García I, Iglesias-de-Sena H, Martín-Rodero H, Herrera C, et al. Association between occupational exposure to wood dust and cancer: a systematic review and meta-analysis. PLoS One 2015;10(7):e0133024. 26191795.ArticlePubMedPMC
- 39. Basomba A, Burches E, Almodovar A, de Rojas DH. Occupational rhinitis and asthma caused by inhalation of Balfourodendron riedelianum (Pau Marfim) wood dust. Allergy 1991;46(4):316–318. 1897693.ArticlePubMed
- 40. Booth BH, LeFoldt RH, Moffitt EM. Wood dust hypersensitivity. J Allergy Clin Immunol 1976;57(4):352–357. 1262610.ArticlePubMed
- 41. Correale CE, Marks JG Jr. Contact dermatitis in a woodworker. Am J Contact Dermat 2002;13(1):42–44. 11887106.ArticlePubMed
- 42. Sripaiboonkij P, Phanprasit W, Jaakkola MS. Respiratory and skin effects of exposure to wood dust from the rubber tree Hevea brasiliensis
. Occup Environ Med 2009;66(7):442–447. 19188201.ArticlePubMed
- 43. Wilhelmsson B, Jernudd Y, Ripe E, Holmberg K. Nasal hypersensitivity in wood furniture workers. An allergological and immunological investigation with special reference to mould and wood. Allergy 1984;39(8):586–595. 6528953.ArticlePubMed
- 44. Wilhelmsson B, Jernudd Y, Ripe E, Holmberg K. Nasal hypersensitivity in wood furniture workers. Rhinology 1985;23(4):297–302. 4081526.ArticlePubMed
- 45. Wilhelmsson B, Lundh B, Drettner B, Stenkvist B. Effects of wood dust exposure and diethylnitrosamine. A pilot study in Syrian golden hamsters. Acta Otolaryngol 1985;99(1-2):160–171. 3976389.ArticlePubMed
- 46. Wilhelmsson B, Hellquist H, Olofsson J, Klintenberg C. Nasal cuboidal metaplasia with dysplasia. Precursor to adenocarcinoma in wood-dust-exposed workers? Acta Otolaryngol 1985;99(5-6):641–648. 4024915.PubMed
- 47. Cormier Y, Mérlaux A, Duchaine C. Respiratory health impact of working in sawmills in eastern Canada. Arch Environ Health 2000;55(6):424–430. 11128881.ArticlePubMed
- 48. Rastogi SK, Gupta BN, Husain T, Mathur N. Respiratory health effects from occupational exposure to wood dust in sawmills. Am Ind Hyg Assoc J 1989;50(11):574–578. 2596398.PubMed
- 49. Bhattacharjee JW, Zaidi SH. In vitro and in vivo studies of organic dusts. Ann Occup Hyg 1982;26(1-4):635–644. 7181293.ArticlePubMed
- 50. Bhattacharjee JW, Dogra RK, Lal MM, Zaidi SH. Wood dust toxicity: in vivo and in vitro studies. Environ Res 1979;20(2):455–464. 546651.ArticlePubMed
- 51. Määttä J, Lehto M, Leino M, Tillander S, Haapakoski R, Majuri ML, et al. Mechanisms of particle-induced pulmonary inflammation in a mouse model: exposure to wood dust. Toxicol Sci 2006;93(1):96–104. 16740616.ArticlePubMed
- 52. Vallières E, Pintos J, Parent ME, Siemiatycki J. Occupational exposure to wood dust and risk of lung cancer in two population-based case-control studies in Montreal, Canada. Environ Health 2015;14(1):1. 25564290.PubMedPMC
- 53. Lai PS, Christiani DC. Long-term respiratory health effects in textile workers. Curr Opin Pulm Med 2013;19(2):152–157. 23361196.ArticlePubMedPMC
- 54. Mandryk J, Alwis KU, Hocking AD. Effects of personal exposures on pulmonary function and work-related symptoms among sawmill workers. Ann Occup Hyg 2000;44(4):281–289. 10831732.ArticlePubMed
- 55. Maciejewska A, Wojtczak J, Bielichowska-Cybula G, Domańska A, Dutkiewicz J, Mołocznik A. Biological effect of wood dust. Med Pr 1993;44(3):277–288. 8231799.PubMed
, Philip Quartey2
, Abdala Mumuni Ussif3
, Niklas Ricklund4
, Daniel Lawer Egbenya5
, Gideon Akuamoah Wiafe5
, Korantema Mawuena Tsegah2
, Akua Karikari2
, Håkan Löfstedt6
, Francis Tanam Djankpa5