Regular consumption of fatty fish rich in n-3 PUFA such as DHA and EPA protects against CVD risk(Reference Balk, Lichtenstein and Chung1, Reference Burillo, Martin-Fuentes and Mateo-Gallego2) and possibly against cognitive decline(Reference Morris, Evans and Bienias3–Reference Fotuhi, Mohassel and Yaffe5). Several epidemiological studies have suggested that higher n-3 PUFA concentrations in plasma or erythrocytes are associated with a lower risk of ageing-associated cognitive decline(Reference Beydoun, Kaufman and Satia6–Reference Samieri, Feart and Proust-Lima9). However, carriers (E4+) of the apoE ε4 allele, the most important genetic risk factor for Alzheimer's disease(Reference Corder, Saunders and Strittmatter10), seem not to be protected against cognitive decline by the consumption of fish(Reference Huang, Zandi and Tucker11). Furthermore, higher erythrocyte n-3 PUFA are not associated with better cognitive function in E4+(Reference Whalley, Deary and Starr12).
DHA is a major structural component of brain membranes and is essential in neuronal development and repair, neurotransmission(Reference Horrocks and Yeo13), cell signalling and anti-inflammatory processes(Reference Calon, Lim and Yang14, Reference Bouwens, van de Rest and Dellschaft15). Synthesis of EPA and DHA from α-linolenic acid (ALA) is extremely limited in humans(Reference Plourde and Cunnane16), so it is advantageous that preformed EPA and DHA be present in the diet. The concentration of DHA in plasma usually follows a logarithmic distribution with dietary DHA intake(Reference Castellano, Chouinard-Watkins and Brenna17, Reference Cunnane, Chouinard-Watkins and Castellano18), but E4+ have a lower plasma response to n-3 PUFA supplementation compared with non-carriers of E4 (E4 − )(Reference Plourde, Vohl and Vandal19). Indeed, after receiving 3 g/d of EPA+DHA for 6 weeks, DHA concentration in plasma TAG increased by 75 % in E4+, whereas in E4 − , the increase was 240 %(Reference Plourde, Vohl and Vandal19). Thus, E4+ appear to have altered DHA metabolism when given an n-3 PUFA supplement.
There are two principal ways to assess DHA metabolism in human subjects: by perturbing plasma DHA with a DHA supplement or using isotopically labelled DHA. An oral dose of uniformly labelled carbon 13 [13C]DHA(Reference Le, Fraser and Gardner20) is a precise and sensitive tool to evaluate the distribution of DHA in plasma and β-oxidation over time. [13C]DHA metabolism in human subjects was first reported more than a decade ago(Reference Brenna21–Reference Lemaitre-Delaunay, Pachiaudi and Laville23). In one study, the authors gave a single oral dose between 250 and 280 mg [13C]DHA in the form of a TAG to three healthy men(Reference Brossard, Croset and Pachiaudi22). [13C]DHA levels reached a maximum 2 h post-dose in plasma TAG and the apparent retroconversion of [13C]DHA to [13C]EPA was estimated at 1·4 % of the total plasma concentration of [13C]DHA. Recently, we gave 50 mg [13C]DHA in the form of a methyl ester to six young and six elderly participants(Reference Plourde, Chouinard-Watkins and Vandal24) and showed that 4 h after the [13C]DHA intake, the elderly had a fourfold higher [13C]DHA concentration in plasma total lipids compared with the young participants(Reference Plourde, Chouinard-Watkins and Vandal24).
Using [13C]DHA, the objective of the present study was to evaluate whether DHA metabolism is different in E4+v. E4 − . We report the distribution of [13C]DHA in plasma total lipids, its apparent retroconversion to EPA detected in plasma total lipids, the β-oxidation of [13C]DHA recovered in breath in the form of 13CO2, and plasma and the whole-body half-life of [13C]DHA.
Methods
A total of forty participants over 50 years of age were recruited between January 2010 and April 2011 in the Eastern Townships of Quebec, Canada. All participants completed the thirty-item Montreal Cognitive Assessment test for baseline cognitive status(Reference Nasreddine, Phillips and Bedirian25). Participants were all non-smokers, and free of dementia or diabetes. They did not have a diagnosis of cancer in the past 6 months, liver or renal disease, uncontrolled hyper- or hypothyroidism, autoimmune disorder, elevated markers of inflammation or low serum albumin. Anyone consuming n-3 PUFA capsules was excluded. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Human Ethics Research Committee of the Health and Social Sciences Center – Sherbrooke University Geriatrics Institute, which is the committee mandated to oversee human experimentation at our institution. Written informed consent was obtained from all participants. The study is registered at www.clinicaltrials.gov (NCT01577004).
Tracer study design
The [13C]DHA used in the present study was uniformly labelled (>98 %) and of high chemical purity (99 % pure). It was synthesised using micro-algae fed with [13C]glucose. Each 40 mg dose of [13C]DHA methyl ester was stored in an individual glass ampoule sealed under Ar(Reference Le, Fraser and Gardner20).
Participants arrived fasted on the morning of the metabolic study day. After collecting baseline blood and breath samples (see details below), the participants received a breakfast composed of two pieces of whole-wheat grain toast with peanut butter, one scrambled egg, one apple, 35 g mozzarella cheese and 250 ml orange juice. The macronutrient composition of this 2805 kJ breakfast was as follows: 25·5 g fat, 78 g carbohydrate and 29 g protein. The 40 mg dose of [13C]DHA was added to a piece of toast. The breakfast was consumed by all participants within 15 min. At 4 h after tracer consumption, the participants were given a lunch composed of lasagne with 200 ml of V8 vegetable juice and a granola bar. The macronutrient composition of this 2093 kJ lunch was as follows: 15 g fat, 88 g carbohydrate and 23 g protein.
[13C]DHA metabolism was monitored in blood and breath samples collected at baseline (0 h) and at 1, 2, 4, 6 and 8 h post-dose. The participants returned to the metabolic unit 1, 7, 14, 21 and 28 d post-dose for blood and breath sample collection. A 28 d follow-up was used since this is the typical amount of time needed for [13C]DHA in plasma to return to baseline(Reference Plourde, Chouinard-Watkins and Vandal24). A catheter was installed in a forearm vein for the collection of blood samples on the first day; later, blood samples were collected using a 5 ml syringe (Becton Dickinson) and transferred into 4 ml EDTA tubes (Becton Dickinson). The tubes were centrifuged at 2300 g for 15 min at 4°C, and plasma was stored in three 0·5 ml Eppendorfs at − 80°C until further analyses.
To assess the appearance of 13CO2 coming from the β-oxidation of [13C]DHA, participants breathed into a device consisting of a perforated plastic bag attached to a mouthpiece (EasySampler; QuinTron Instrument Company) to which an evacuated glass tube was inserted to collect a sample of the exhaled breath(Reference Freemantle, Vandal and Tremblay Mercier26, Reference McCloy, Ryan and Pencharz27).
Analytical methods
Total lipids were extracted from 0·25 ml of plasma using the method described by Folch et al. (Reference Folch, Lees and Sloane Stanley28). Heptadecanoate was added as an internal standard for quantification of fatty acids. To remove cholesterol, the total lipid extract was then saponified using 3 ml of 1 m-KOH–methanol and the mixture was heated at 90°C for 1 h. Transmethylation of the resulting NEFA into fatty acid methyl esters was performed using 14 % boron trifluoride–methanol (Sigma-Aldrich). Fatty acid methyl esters were analysed using a gas chromatograph (model 6890; Agilent) equipped with a 50 m BPX-70 fused capillary column (SGE). Injection and flame ionisation detection were performed at 250°C with the following oven temperature programme: 50°C for 2 min, increased by 20°C/min to 170°C for 15 min and finally increased by 5°C/min to 210°C for 7 min. He gas was used as a carrier and the inlet pressure was 233 kPa at 50°C. The identity of individual fatty acids was determined using standard mixtures of fatty acids (NuChek 68A, NuChek 411 and NuChek 455; NuChek Prep, Inc.) and a custom mixture of SFA.
[13C]DHA enrichment analysis in plasma total lipids was performed using GC–combustion–isotope ratio MS, as described previously(Reference Goodman and Brenna29). 13C/12C post-dose was compared with baseline 13C/12C (pre-dose) to calculate the δ (per mil) values that were designated thereafter as atom per cent excess. Calculations of [13C]DHA (nmol/ml) and [13C]EPA (pmol/ml) from the atom per cent excess values were performed according to Brossard et al. (Reference Brossard, Croset and Pachiaudi22).
Enrichment of 13C in breath CO2 after [13C]DHA consumption was analysed by isotope ratio MS (ABCA, Sercon Limited), as described previously(Reference McCloy, Ryan and Pencharz27). He gas (Praxair) was used as a carrier and 5 % CO2/N2 as the reference gas. The percentage dose of [13C]DHA recovered in breath as 13CO2 was calculated as described previously(Reference Freemantle, Vandal and Tremblay Mercier26), except that basal metabolism was evaluated using indirect calorimetry (CCM/D; Medgraphics Corporation) to measure the volume of CO2 and O2 exhaled by the participants over 30 min(Reference Choquette, Chuin and Lalancette30). Cumulative β-oxidation of [13C]DHA was calculated from the AUC of the percentage dose recovered at each time point (GraphPad Prism 5 software; GraphPad Software, Inc.).
[13C]DHA half-life in plasma was calculated using RxKinetics online software (RxKinetics; www.rxkinetics.com). The values of two E4 − participants were excluded because [13C]DHA concentrations in plasma were not available for time points 14, 21 and 28 d. [13C]DHA half-life in the whole body was estimated for each participant using cumulative 13CO2 data. For three E4+ and twenty-five E4 − not reaching a cumulative β-oxidation of 50 % 28 d post-dose, it was assumed from the cumulative 13CO2 data of the other participants that beyond 28 d, the curve would be linear (see Fig. 1(d)). Therefore, using the cumulative 13CO2 data at times 1, 7, 14, 21 and 28 d post-dose, a linear equation in the form of y= mx+b, where m is the slope and b is the y value when x= 0, was calculated for each participant to estimate the time needed (x) to reach 50 % (y) of cumulative β-oxidation of [13C]DHA recovered as 13CO2. As a result, five E4 − were excluded because β-oxidation of [13C]DHA recovered as 13CO2 reached a plateau of < 50 % 7 d post-dose, so it was not possible to estimate the whole-body [13C]DHA half-life. Correlations between [13C]DHA concentration in plasma and the percentage dose of [13C]DHA recovered as breath 13CO2 were performed using all time points for all participants (n 58 for E4+ and n 314 for E4 − ). Baseline values of 13C in plasma DHA and the percentage of 13C in CO2 were removed before the correlations between [13C]DHA concentration in plasma and the percentage dose of [13C]DHA recovered as breath 13CO2 since these values were standardised at zero in our calculations. The slopes of the linear regression between [13C]DHA concentration in plasma and the percentage dose of [13C]DHA recovered as breath 13CO2 was calculated and compared between E4+ and E4 − .

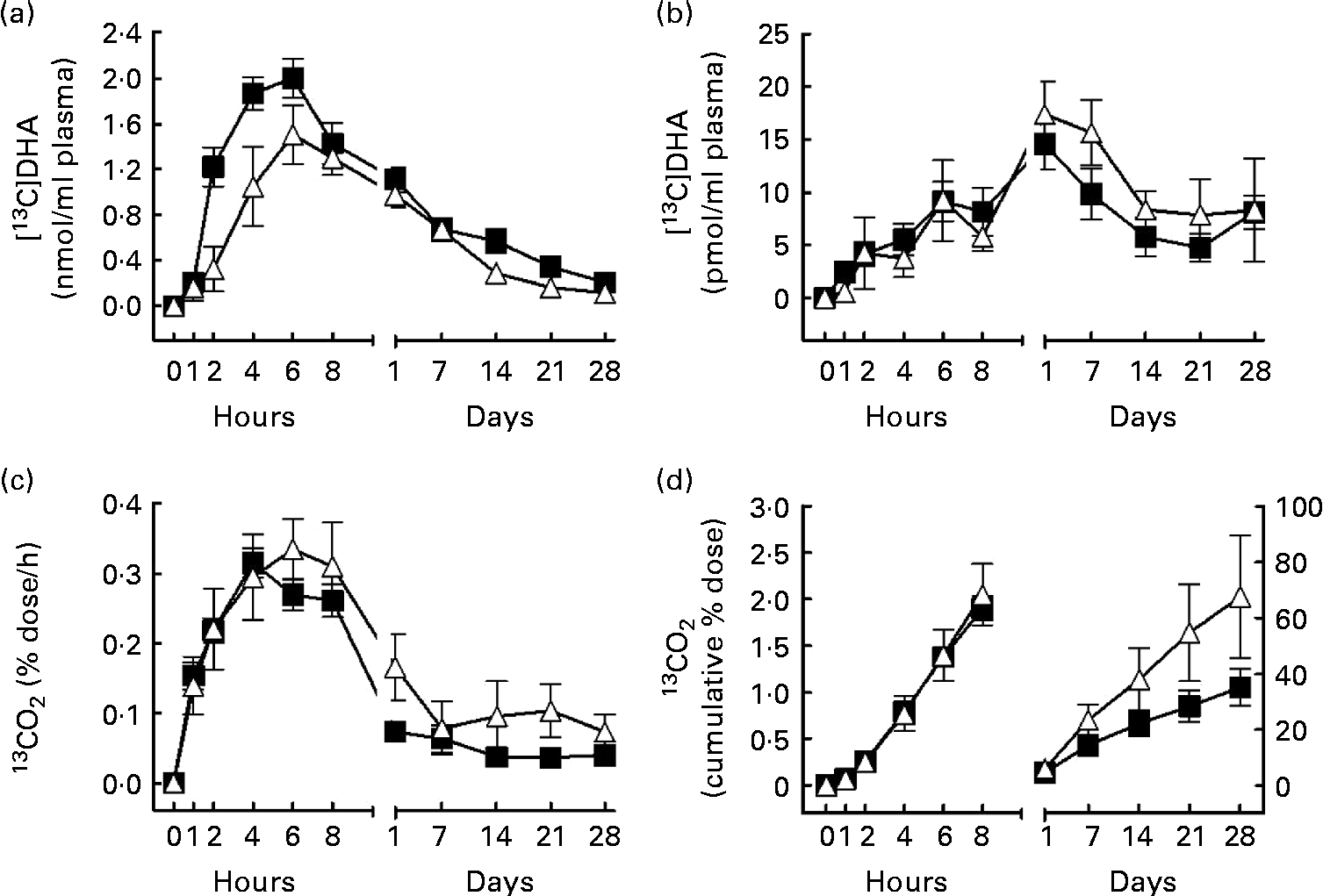
Fig. 1 [13C]DHA metabolism over 28 d after an oral dose of 40 mg [13C]DHA in apoE ε4 carriers (E4+, Δ, n 6) and non-carriers (E4 − , ■, n 34). (a) [13C]DHA concentration (nmol/ml) in plasma total lipids, (b) [13C]DHA apparent retroconversion into [13C]EPA in plasma total lipids, (c) the percentage dose of [13C]DHA recovered/h as 13CO2 in breath and (d) the cumulative percentage dose of [13C]DHA recovered as 13CO2 over 28 d of follow-up. In (d), the left curves follow the left y-axis, whereas the right curves follow the right y-axis. The estimated slope of the right curve (m) was 0·09 (sem 0·03) in E4+v. 0·05 (sem 0·01) in E4 − (P= 0·03). Values are means, with their standard errors represented by vertical bars. There were significant effects for (a) genotype (P= 0·04) and (d) the genotype × time interaction (P= 0·003).
ApoE genotyping
DNA of the participants was extracted from 200 μl of whole blood (QIAmp DNA Blood Mini Kit; Qiagen). The DNA fragment containing the apoE gene (APOE) sequence was amplified by PCR (Perkin Elmer GeneAmp PCR System 2400; Perkin Elmer) using the oligonucleotide primers F6 (5′-TAAGCTTGGCACGGCTGTCCAAGGA-3′) and F4 (5′-ACAGAATTCGCCCCGGCCTGGTACAC-3′), as described previously(Reference Emi, Wu and Robertson31). After amplification, the DNA fragment was digested using HhaI (New England Biolabs Ltd) in order to reveal differential digestion patterns related to the APOE genotype. DNA fragments were then loaded on a 20 % polyacrylamide gel for migration at 220 V for 3 h and the fragments were revealed using ethidium bromide(Reference Hixson and Vernier32).
Data expression and statistics
Sample size was based on a calculation that the maximum concentration of [13C]DHA in plasma total lipids that would be reached in the postprandial period would be 0·9 nmol/ml(Reference Plourde, Chouinard-Watkins and Vandal24). From our previous study(Reference Plourde, Chouinard-Watkins and Vandal24), we estimated that a twofold difference in plasma [13C]DHA concentration would be observed between E4+ and E4 − in the postprandial period(Reference Plourde, Vohl and Vandal19). Therefore, the sample size required to detect this difference using a 5 % significance level and a power of 80 % was six subjects per group(33). We based our sample size calculation only on plasma [13C]DHA concentration because DHA in plasma is one of the best documented biomarkers of the difference between E4+ and E4 − (Reference Plourde, Vohl and Vandal19). Moreover, since pre-screening for E4+ is not permitted at our institution, we therefore enrolled forty participants to recruit at least six E4+ on the assumption that E4+ frequency is approximately 15–25 %(Reference Garenc, Aubert and Laroche34, Reference Bullido, Artiga and Recuero35) in the general population, heterozygous and homozygous E4+ combined. Data are shown as means with their standard errors.
Statistics in Fig. 1 were performed using the PROC MIXED procedure implemented in SAS since some participants had missing data (SAS 9.2; SAS Institute)(Reference Littell, Henry and Ammerman36). This procedure was used instead of a classical two-way ANOVA to optimise the use of all data over time and maintain statistical power. The PROC MIXED procedure allows testing for the effect of time as a repeated measure, genotype as a fixed factor (E4+v. E4 − ) and the interaction genotype × time. Student's t tests were performed on Fig. 2, Table 1 and Table 2 to detect significant differences between E4+ and E4 − (SPSS 17.0; SPSS, Inc.). The correlation coefficient (R) of the correlations between plasma [13C]DHA and [13C]DHA recovered/h as breath 13CO2 was performed using the bivariate correlation program in SPSS (Fig. 3). To account for potential confounding factors, correlation analyses were performed between [13C]DHA recovered/h as breath 13CO2 and baseline characteristics such as age, sex, cholesterol levels, glucose levels and medications. Statistically significant correlations were included in a multiple linear regression model. The slopes (β) of the linear regression between plasma [13C]DHA and [13C]DHA recovered/h as 13CO2 were compared between E4+ and E4 − . For cumulative β-oxidation of [13C]DHA recovered as 13CO2 between E4+ and E4 − , a simple linear regression model was used to compare the slope (m) between 1 d and 28 d post-dose. Statistical significance was set at P≤ 0·05.

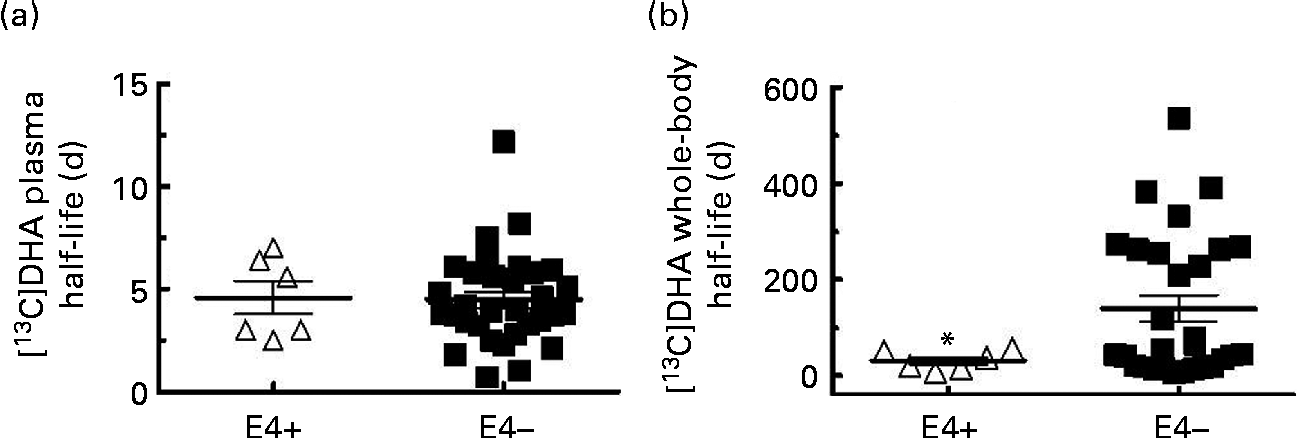
Fig. 2 [13C]DHA half-life in (a) plasma and (b) in the whole body in apoE ε4 carriers (E4+, Δ, n 6) and non-carriers (E4 − , ■, n 32 for (a) and n 29 for (b)). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different compared with E4 − (P≤ 0·05).
Table 1 Baseline characteristics of apoE ε4 allele carriers (E4+) and apoE ε4 non-carriers (E4−) (Mean values with their standard errors)

TSH, thyroid-stimulating hormone; CRP, C-reactive protein; MoCA, Montreal Cognitive Assessment.
* Score out of a maximum of 30.
† The number of subjects in each group receiving each medication.
‡ Anti-hypertensive agents include angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, β-blockers, Ca channel blockers and diuretics.
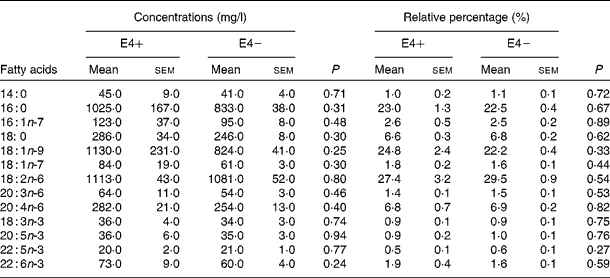
Table 2 Fatty acid concentration (mg/l) and percentage in plasma total lipids of apoE ε4 allele carriers (E4+) (n 6) and apoE ε4 non-carriers (E4−) (n 34) at baseline (Mean values with their standard errors)


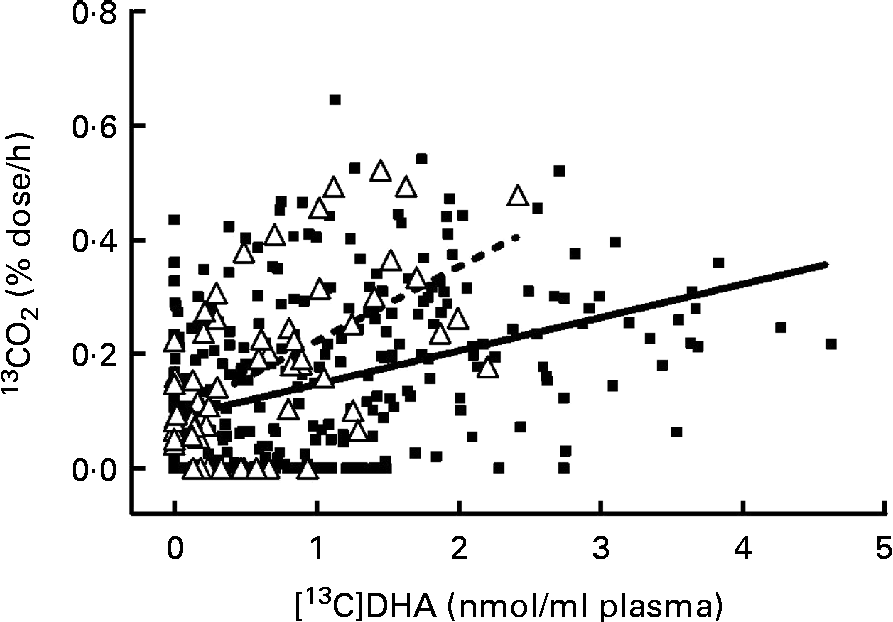
Fig. 3 Linear regression between [13C]DHA concentration (nmol/ml) in plasma total lipids and the percentage dose of [13C]DHA recovered/h as 13CO2 in the breath of apoE ε4 carriers (E4+, Δ, ![]() , n 58, R 0·56) and non-carriers (E4 − , ■,
, n 58, R 0·56) and non-carriers (E4 − , ■, ![]() , n 314, R 0·39) over 28 d of follow-up. The linear regression model had a slope (β) of 0·13 (sem 0·03) in E4+v. 0·06 (sem 0·01) in E4 − (P< 0·001).
, n 314, R 0·39) over 28 d of follow-up. The linear regression model had a slope (β) of 0·13 (sem 0·03) in E4+v. 0·06 (sem 0·01) in E4 − (P< 0·001).
Results
In the present study, six participants were E4+ (five E3/E4 and one E2/E4, two men and four women) and thirty-four were E4 − (twenty-eight E3/E3 and six E2/E3, twelve men and twenty-two women). In E4+, the mean age was 68·0 (sem 3·3) years, whereas it was 72·4 (sem 1·5) years in E4 − (NS; Table 1). There was no difference in baseline characteristics between E4+ and E4 − (Table 1) and between men and women (data not shown). The participants' score on the thirty-item Montreal Cognitive Assessment test was 26·1 (sem 0·5) (maximum score of 30), indicating that they were cognitively healthy at baseline(Reference Nasreddine, Phillips and Bedirian25). In E4+ at baseline, the mean plasma DHA concentration was 73 (sem 9) mg/l, which was equivalent to 1·9 (sem 0·4) % of plasma total fatty acids, whereas in E4 − , DHA concentration was 60 (sem 4) mg/l, which was equivalent to 1·6 (sem 0·1) % of plasma total fatty acids (Table 2). There was no difference in fasting plasma fatty acid compositions between E4+ and E4 − (Table 2).
[13C]DHA metabolism in apoE ε4 allele carriers v. apoE ε4 non-carriers
The PROC MIXED procedure detected no genotype × time interaction with plasma [13C]DHA (Fig. 1(a)). Nevertheless, a genotype effect was detected for plasma [13C]DHA, such that in E4+, [13C]DHA in plasma total lipids from 1 h to 28 d post-dose was 31 % lower compared with E4 − (mean 0·66 (sem 0·14) nmol/ml in E4+v. 0·96 (sem 0·11) nmol/ml in E4 − , P= 0·04; Fig. 1(a)). In both groups, [13C]DHA peaked in plasma total lipids 6 h after tracer intake; in E4+, the maximum value of [13C]DHA was 1·5 (sem 0·3) nmol/ml, whereas it was 2·0 (sem 0·2) nmol/ml in E4 − (NS; Fig. 1(a)).
The apparent retroconversion of [13C]DHA into [13C]EPA peaked 1 d post-dose, but was not different between E4+ and E4 − and no genotype × time interaction was detected for plasma [13C]EPA concentration (Fig. 1(b)). In E4+, [13C]EPA concentration in plasma total lipids reached a maximum of 17·4 (sem 3·1) pmol/ml, representing 1·2 % of the peak plasma [13C]DHA concentration, whereas in E4 − , [13C]EPA concentration in plasma total lipids reached a maximum of 14·4 (sem 2·4) pmol/ml, representing 0·7 % of the peak [13C]DHA concentration (NS; Fig. 1(b)).
The percentage dose of [13C]DHA recovered/h as 13CO2 did not differ at any time point between E4+ and E4 − over the 28 d of the study (Fig. 1(c)). On the other hand, a genotype × time interaction was detected with regard to cumulative β-oxidation of [13C]DHA recovered as 13CO2 (P= 0·003; Fig. 1(d)). Moreover, in E4+, the slope of the cumulative 13CO2 line (m) between 1 and 28 d post-dose was 80 % steeper than in E4 − (m= 0·09 (sem 0·03) in E4+v. m= 0·05 (sem 0·01) in E4 − , P= 0·03; Fig. 1(d)). In E4+, cumulative β-oxidation of [13C]DHA recovered as 13CO2 reached a maximum of 68 (sem 22) % 28 d post-dose, whereas it reached 35 (sem 7) % in E4 − (NS; Fig. 1(d)).
The [13C]DHA half-life in plasma was 4·6 (sem 0·8) d in E4+ and 4·5 (sem 0·4) d in E4 − (NS; Fig. 2(a)). The whole-body [13C]DHA half-life was heterogeneous and was 77 % lower in E4+ compared with E4 − (32 (sem 8) d in E4+v. 140 (sem 28) d in E4 − , P= 0·001; Fig. 2(b)). In two E4+, the mean whole-body [13C]DHA half-life was 53 d, whereas in four other carriers, it was 21 d (Fig. 2(b)). In eleven E4 − , the whole-body [13C]DHA half-life was >200 d, whereas in fourteen other E4 − , it was < 50 d and in four E4 − , it was between 50 and 200 d (Fig. 2(b)).
Correlation between [13C]DHA concentration in plasma and the percentage dose of [13C]DHA recovered/h as 13CO2
In E4+ and E4 − , the percentage dose of [13C]DHA recovered/h as 13CO2 correlated positively with [13C]DHA concentration in plasma (R 0·56 in E4+ and R 0·39 in E4 − , P< 0·001 for both; Fig. 3). The percentage dose of [13C]DHA recovered/h as 13CO2 also correlated with the levels of LDL-cholesterol and was associated with the use of a statin, so these potential confounding factors were included in the multivariate linear regression model. There was a positive interaction between genotype and linear regression of the percentage dose of [13C]DHA recovered/h as 13CO2 (y) with [13C]DHA concentration in plasma (x), and this interaction remained significant when accounting for confounding factors (P< 0·001; Fig. 3). The slope of the linear regression (β) was 117 % steeper in E4+ compared with E4 − (β = 0·13 (sem 0·03) in E4+v. β = 0·06 (sem 0·01) in E4 − , P< 0·001).
Discussion
These results demonstrate that [13C]DHA metabolism is disturbed in E4+ compared with E4 − since E4+ had a 31 % lower mean concentration of [13C]DHA in plasma total lipids over time, but increased β-oxidation between 1 and 28 d post-dose. This difference may be due, at least in part, to the key role of apoE in postprandial plasma lipoprotein and lipid metabolism(Reference Hooijmans and Kiliaan37). ApoE has a high affinity for the LDL receptor that is involved in lipoprotein clearance from the plasma, notably chylomicron remnants and VLDL(Reference Mahley and Ji38). E4+ have a lower concentration of apoE protein in plasma(Reference Bahri, Esteban and Moral39), but with proportionally more apoE in VLDL and less in HDL compared with homozygous carriers of apoE ε3(Reference Gregg, Zech and Schaefer40). Therefore, clearance of VLDL in E4+ is potentially more rapid since this process relies partly on the binding of apoE protein with the LDL receptor(Reference Gregg, Zech and Schaefer40). Fatty acids travel in the blood mostly via lipoproteins, so a more rapid VLDL turnover potentially enhances [13C]DHA clearance from the plasma, thereby supporting the present observation of lower plasma [13C]DHA concentrations in E4+. Moreover, [13C]DHA was ingested in the form of a methyl ester. Hence, it is possible that the observed differences in [13C]DHA concentrations between E4+ and E4 − might be partly due to a difference in the cleavage capacity of this form of DHA in E4+, although, to our knowledge, no study has evaluated this question.
Several studies have shown that E4+ have higher plasma TAG than E4 − in the postprandial state(Reference Carvalho-Wells, Jackson and Gill41, Reference Kobayashi, Saito and Taira42), and that there is an age × APOE genotype interaction with regard to TAG metabolism after an oral fat load(Reference Carvalho-Wells, Jackson and Gill41, Reference Reznik, Morello and Pousse43). Postprandially, E4+ over 50 years old had a higher AUC for plasma TAG concentrations compared with E4 − , whereas this difference was absent between younger E4+ and E4 − (Reference Carvalho-Wells, Jackson and Gill41). In the present study, all participants were aged >50 years old, so we would anticipate a higher DHA concentration in the postprandial state because of higher postprandial TAG levels in E4+. However, there was no difference between E4+ and E4 − in total postprandial DHA expressed either in mg/l or in relative percentage to other fatty acids. Nevertheless, TAG levels during the postprandial state were not evaluated in the present study, so we cannot confirm the results obtained by Carvalho-Wells et al. (Reference Carvalho-Wells, Jackson and Gill41) with regard to TAG concentrations in the postprandial state between E4+ and E4 − .
We report here for the first time the β-oxidation of [13C]DHA in E4+ and E4 − . E4+ had higher cumulative β-oxidation of [13C]DHA than E4 − between 1 and 28 d post-dose (Fig. 1(d)). The estimated slope (m) of the cumulative β-oxidation of [13C]DHA between 1 and 28 d post-dose was 80 % steeper in E4+ than in E4 − (Fig. 1(d)), suggesting a higher rate of the β-oxidation of [13C]DHA in E4+ compared with E4 − . This result could help explain the lower plasma [13C]DHA in E4+ compared with E4 − . The cumulative β-oxidation of [13C]DHA 24 h post-dose was 6 % in E4+ and 5 % in E4 − (NS; Fig. 1(d)), which was nearly 75 % lower compared with other common dietary fatty acids such as oleic acid (29 %), linoleic acid (21 %) or ALA (31 %)(Reference McCloy, Ryan and Pencharz27). This suggests that in humans habitually consuming low levels of DHA, DHA is efficiently conserved, probably because of its structural importance in cell membranes(Reference Litman, Niu and Polozova44) and as a precursor to signalling molecules derived from DHA, notably resolvins and protectins(Reference Serhan, Clish and Brannon45).
Plasma [13C]DHA correlated with the percentage dose of [13C]DHA recovered/h as 13CO2 in both E4+ and E4 − , but the slope (β) of this relationship was 117 % steeper in E4+ than in E4 − (P< 0·001; Fig. 3). Thus, for a given plasma concentration of [13C]DHA, 13CO2 was higher in E4+ than in E4 − , showing more rapid β-oxidation of DHA. This difference in retention v. oxidation of DHA in E4+ is consistent with our previous report that the increase in plasma DHA after supplementing with EPA+DHA was lower in E4+ than in E4 − (Reference Plourde, Vohl and Vandal19). β-Oxidation of DHA is thought to be mainly conducted in peroxisomes(Reference Liang, Zhu and Schulz46), but the relative contribution of peroxisomal v. mitochondrial β-oxidation to the whole-body production of 13CO2 from [13C]DHA in humans is unknown. To the best of our knowledge, there are currently no available data supporting a potential role of APOE4 polymorphism on the expression and/or activity of these peroxisomal enzymes β-oxidising DHA. A recent review by Lizard et al. (Reference Lizard, Rouaud and Demarquoy47) has suggested the potential dysfunction of peroxisomal metabolism in patients with Alzheimer's disease. Since E4+ are more at risk to develop Alzheimer's disease, the present results showing more β-oxidation of [13C]DHA needs further investigation since the APOE genotype may potentially affect Alzheimer's disease risk by affecting the molecular mechanism involved in fatty acid β-oxidation.
The present study is also the first to estimate plasma and whole-body half-lives of [13C]DHA. In previous studies, calculation of [13C]DHA half-life was not possible since a follow-up of < 72 h did not provide enough time for plasma [13C]DHA to return to baseline(Reference Brossard, Croset and Pachiaudi22). In our previous study(Reference Plourde, Chouinard-Watkins and Vandal24), β-oxidation of [13C]DHA was monitored over 7 d post-dose and gave a rough estimate of the [13C]DHA whole-body half-life of about 10 d, which is 66 % less than our current estimate for E4+ and 90 % less for E4 − (Fig. 2). However, in the present study, β-oxidation of [13C]DHA was followed over 28 d and the number of participants was higher than previously(Reference Brossard, Croset and Pachiaudi22–Reference Plourde, Chouinard-Watkins and Vandal24), thus permitting a more accurate estimate of the whole-body half-life of [13C]DHA. We estimated that the whole-body half-life of [13C]DHA was approximately 25 d more than its plasma half-life in E4+ and >100 d more than its plasma half-life in E4 − . Moreover, the whole-body half-life of [13C]DHA was 77 % lower in E4+ compared with E4 − , corroborating higher β-oxidation of [13C]DHA in E4+. Since the whole-body half-life values of [13C]DHA were highly heterogeneous in the E4 − group, we investigated potential factors besides the APOE genotype that could be associated with a higher or lower whole-body half-life. No association was found between the whole-body half-life of [13C]DHA and age, sex, baseline TAG levels, BMI, baseline DHA or EPA status and medications (data not shown).
The apparent retroconversion of [13C]DHA into [13C]EPA was 1·2 %, which is similar to the level reported previously(Reference Brossard, Croset and Pachiaudi22, Reference Plourde, Chouinard-Watkins and Vandal24). These results suggest that most of the [13C]DHA remain in its native form in human subjects, whereas E4+ tend to have an overall higher β-oxidation of [13C]DHA without producing more [13C]EPA compared with E4 − .
A potential confounding factor worth considering in the present study was whether sex disturbed DHA metabolism, as suggested by other studies(Reference Bakewell, Burdge and Calder48–Reference Burdge and Wootton52), since the results presented here involved men and women pooled together. Previous studies have shown that women tend to have higher DHA and EPA in plasma total lipids compared with men(Reference Bakewell, Burdge and Calder48), and this is potentially because of a higher conversion of ALA to EPA and DHA in women(Reference Burdge49, Reference Burdge and Calder51, Reference Burdge and Wootton53) compared with men(Reference Burdge, Jones and Wootton54). Moreover, using [13C]ALA, a study has reported higher β-oxidation in men compared with women, which was associated with higher 13C enrichment in saturated acids and monounsaturated acids, suggesting a preferential pathway towards ALA degradation in men(Reference Burdge and Wootton52). These sex-specific differences in n-3 PUFA metabolism seem to be in part explained by higher estrogens in pre-menopausal women(Reference Decsi and Kennedy50). In the present study, there was no difference in plasma [13C]DHA, plasma DHA or [13C]DHA half-lives in the whole body or plasma between men and women (data not shown). This is probably because our participants were approximately 71 years old and all the women were postmenopausal. The drop in estrogen levels following menopause(Reference Cauley, Gutai and Kuller55) probably contributed to the lack of sex-specific differences in [13C]DHA metabolism in the present study.
The present study had limitations. The number of E4+ was small when compared with E4 − , but baseline characteristics were similar between the two groups (Table 1). Moreover, sample size calculation indicated that six participants should be enough to detect a significant difference in [13C]DHA metabolism. Pre-screening for E4+ is not permitted at our institution, so the only alternative for recruiting E4+ participants is to run the trial and perform APOE genotyping afterwards. There was no difference in cholesterol levels between E4+ and E4 − , even though other studies have suggested otherwise(Reference Garry, Baumgartner and Brodie56–Reference Giltay, van Reedt Dortland and Nissinen58), but our participants were elderly and three E4+ and fourteen E4 − were on statins during the study. No difference in [13C]DHA appearance in plasma and β-oxidation was observed when comparing the participants taking statins or not (data not shown). Another limitation of the present study was that [13C]DHA was the only fatty acid tracer used to follow precisely its metabolism. Hence, whether E4+ disturbs the metabolism of fatty acids other than DHA cannot be deduced from the present study and will need further work with 13C-labelled fatty acids other than DHA.
Conclusion
Compared with E4 − , E4+ had lower mean plasma [13C]DHA between 1 h and 28 d post-dose, whereas β-oxidation of [13C]DHA was higher between 1 and 28 d post-dose. For a similar level of [13C]DHA in plasma, E4+ had higher 13CO2 in breath and a lower whole-body half-life of [13C]DHA compared with E4 − , suggesting higher [13C]DHA catabolism in E4+. Nevertheless, plasma [13C]DHA half-life was similar between E4+ and E4 − . Therefore, there seems to be no clear relationship between plasma half-life and the kinetics of [13C]DHA metabolism. Given that DHA is important for cardiovascular and brain health, disturbance in [13C]DHA metabolism in E4+ may increase their vulnerability to cognitive decline or other diseases. These results may help explain why no association between plasma DHA and cognition has been observed in E4+. Further studies evaluating [13C]DHA metabolism after a DHA supplement are needed to evaluate whether a high dose of EPA+DHA could return DHA homeostasis in E4+ towards normal.
Acknowledgements
The present study was supported by the Advanced Foods and Materials Network, Research Center on Aging, Fonds de la recherche en santé du Québec for a scholarship and a Junior 1 salary award to M. P., Natural Sciences and Engineering Research Council of Canada, a Canada Research Chair to S. C. C., a Canada Research Chair to M. C. V. on Genomics Applied to Nutrition and Health and the Canada Foundation for Innovation. Conrad Filteau, Martine Fisch and Christine Brodeur-Dubreuil provided excellent technical assistance. We thank Dr Anthony Windust (National Research Council, Ottawa) for synthesising the [13C]DHA.
The authors' contributions were as follows: M. P. and S. C. C. designed the study; R. C.-W. conducted the study; Y. Z., P. L. and J. T. B. conducted the 13C analyses; R. C.-W. and J. T.-M. performed the lipid analysis; M. P., S. C. C., R. C.-W. and J. T. B. analysed the data; R. C.-W. performed the statistical analysis; P. P. and R. C.-W. recruited the participants; M. C. V. performed the APOE genotyping; D. L. performed the cognitive tests; R. C.-W. prepared the first draft of the manuscript. All authors contributed to the writing and reviewing of the paper, and read and approved the final version of the manuscript.
The authors report no conflict of interest.